A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
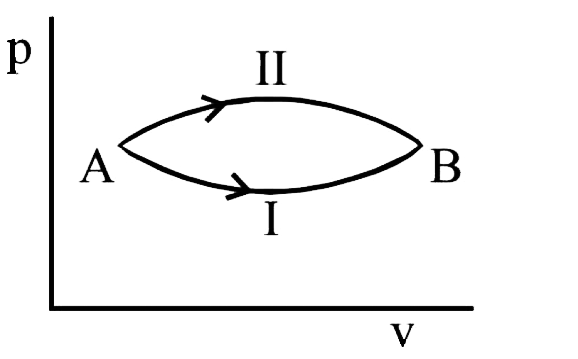
- A system goes from A and B via two processes. I and II as shown in fig...
Text Solution
|
- A system goes from A and B via two processes. I and II as shown in fig...
Text Solution
|
- দুটি পদ্ধতি I ও II-এর মাধ্যমে একটি তন্ত্র A থেকে B -তে যেতে পারে। দুটি...
Text Solution
|
- The figure shows a rotating process. A Oh B The change of intuition in...
Text Solution
|
- किसी गैस की अवस्था A से B तक चित्रानुसार प्रक्रम I तथा II के अनुसार पर...
Text Solution
|
- A system goes from state A to B via two processes I and II, as shown i...
Text Solution
|
- A system goes from A to B via two processes. If deltaU1 and delta U2 a...
Text Solution
|
- A system goes from A to B via two processes I and II as shown in the f...
Text Solution
|
- In the figure given two processes A and B are shown by which a thermod...
Text Solution
|