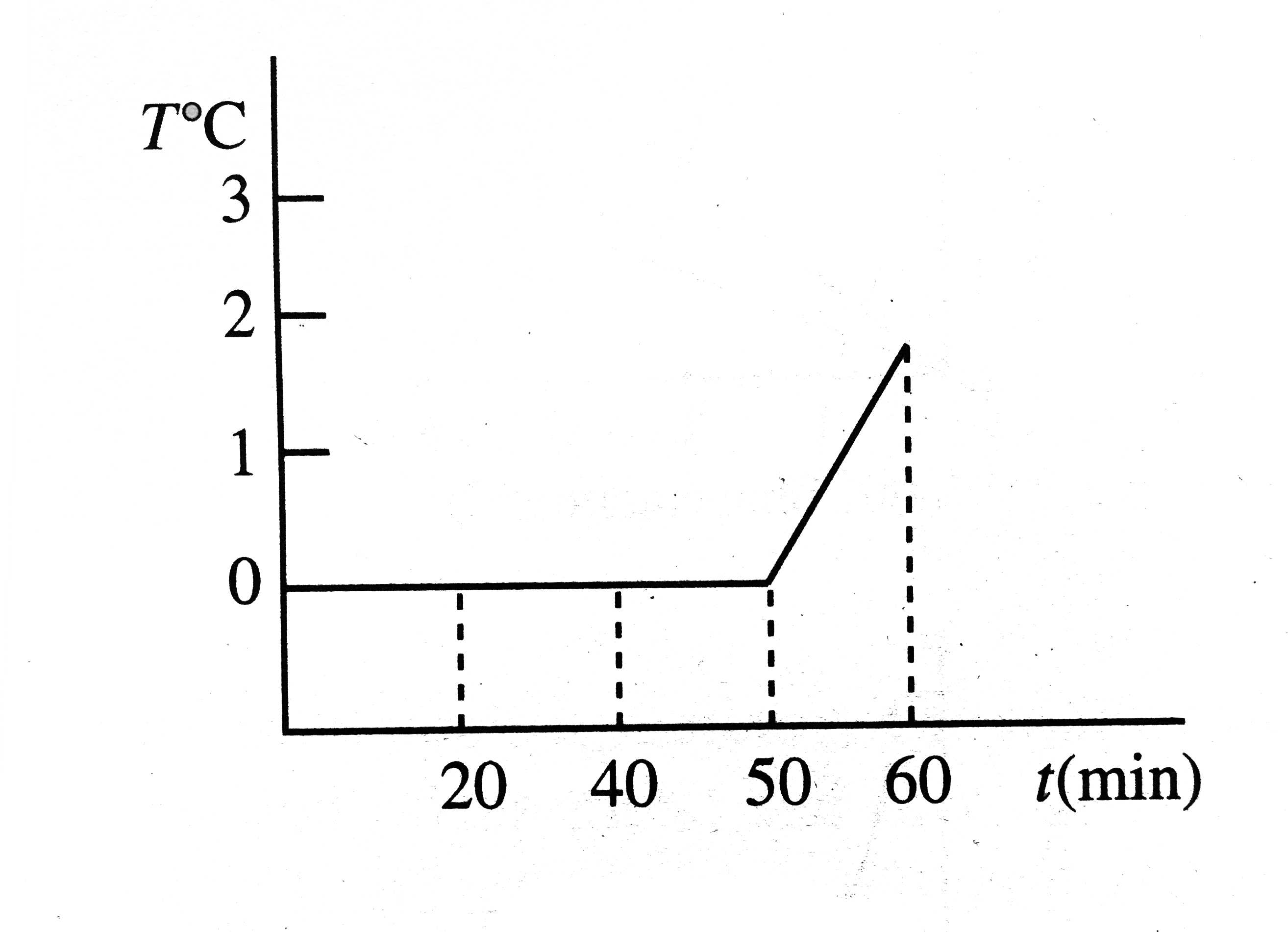A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A cooking vessel on a slow burner contains 5 kg of water and an unknow...
Text Solution
|
- Hot water cools from 60^@C to 50^@C in the first 10 min and to 42^@C i...
Text Solution
|
- A cooking vessel on a slow burner contains 5 kg of water and an unknow...
Text Solution
|
- 10 g of ice at 0^@C is mixed with 100 g of water at 50^@C. What is the...
Text Solution
|
- A container contains 5 kg of water at 0^(@)C mixed to an unknown mass ...
Text Solution
|
- 50 gram of ice at 0^(@)C is mixed with 50 gram of water at 60^(@)C , f...
Text Solution
|
- A body cools from 50^(@)C to 46^(@)C in 5 min and to 40^(@)C in the ne...
Text Solution
|
- 50 g ice at 0^(@)C in kept in an insulating vessel and 50 g water at 1...
Text Solution
|
- Hot water cools from 60^(@)C" to "50^(@)C in the first 10 min and to 4...
Text Solution
|