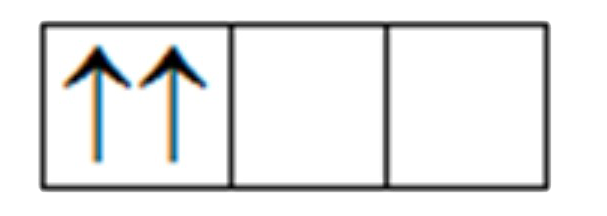
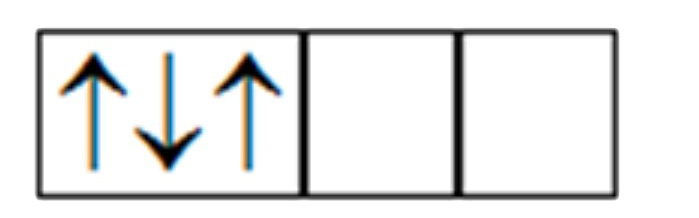
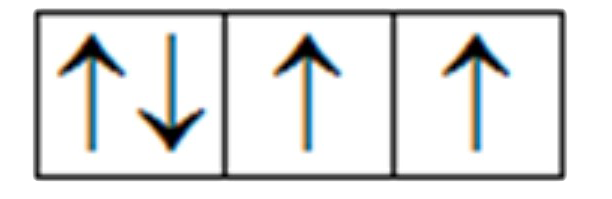
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is not according to the Pauli exclusion princip...
Text Solution
|
- PAULI EXCLUSION PRINCIPLE AND HUND'S RULE
Text Solution
|
- In absence of Pauli exclusion principle , the elecrronic configureatio...
Text Solution
|
- Which of the following is a violation of the Pauli exclusion principle...
Text Solution
|
- Which of the following violates the Pauli exclusion principle :-
Text Solution
|
- the pauli exclusion principle states that :
Text Solution
|
- पाउली का अपवर्जन सिद्धान्त क्या है ? इसके अनुप्रयोग लिखिए।
Text Solution
|
- Explain Pauli's Exclusion principle with an example.
Text Solution
|
- Pauli Exclusion Principle | Aufbau Rule | Hund's Rule
Text Solution
|