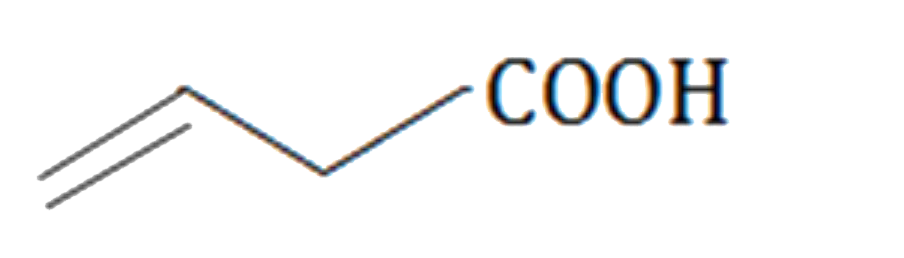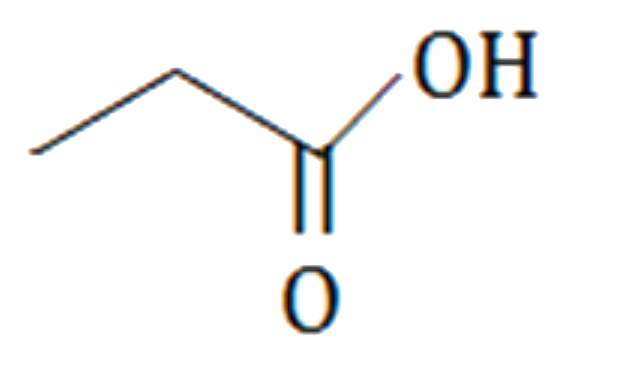A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the compounds shown below will react with diethyl malonate in...
Text Solution
|
- The compound formed when malonic acid is heated with urea, is :
Text Solution
|
- An organic compound [A] is C(5)H(8)O(3). On heating with soda lime, it...
Text Solution
|
- An organic compound [A] is C(5)H(8)O(3). On heating with soda lime, it...
Text Solution
|
- Name the product formed during the decarboxylation of malonic acid.
Text Solution
|
- Which of the aromatic compounds reacts fastest with methoxide ion?
Text Solution
|
- Which of the compounds shown below will react with diethyl malonate in...
Text Solution
|
- Which of the aromatic compounds reacts fastest with methoxide ion?
Text Solution
|
- Which one is formed when Benzaldehyde reacts with Malonic acid in the ...
Text Solution
|