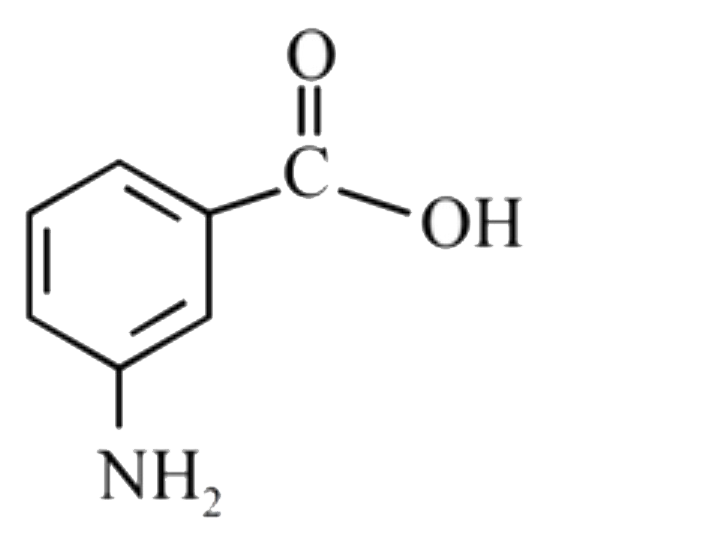
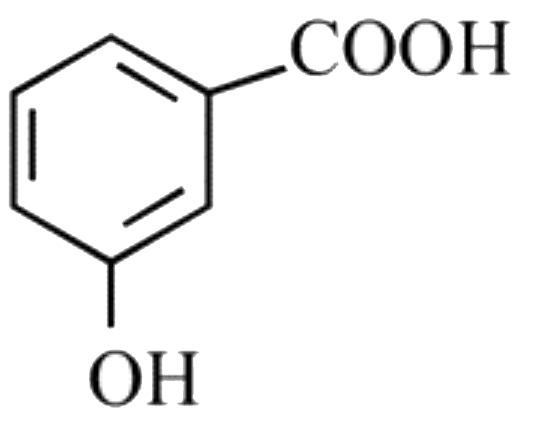
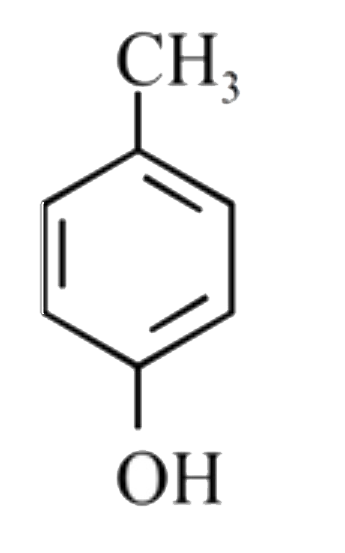
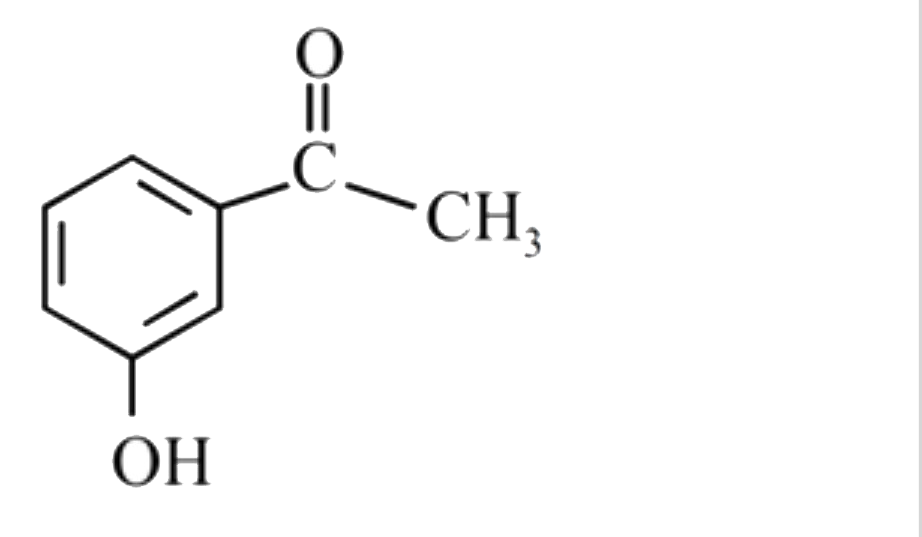
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- What is the major product (Z) of the reaction? PhCOOH underset(H2SO4...
Text Solution
|
- overset (NaBH4) (rarr) overset ((i) H2O^+)underset (Delta) (rarr) A, A...
Text Solution
|
- pH-underset(underset(Me)|)overset(overset(OH)|)(C)-underset(underset(M...
Text Solution
|
- Ph-NO(2) overset(Sn//HCl)rarr underset("HCl, " 0^(@)C-5^(@)C)overset(N...
Text Solution
|
- underset(Zn)overset(O(3))(rarr) (A) underset(Delta)overset(KOH)(rarr) ...
Text Solution
|
- Identify the product (E ) in the following sequence of reactions. o...
Text Solution
|
- What is the major product (Z) of the reaction? PhCOOH underset(H2SO4)o...
Text Solution
|
- C6H6 underset(H2SO4)overset(HNO3)(rarr)X underset(FeBr3)overset(Br2)ra...
Text Solution
|
- In the reaction C2H5NH2 overset(NaNO2)underset(HCl)rarr [X] , X is
Text Solution
|