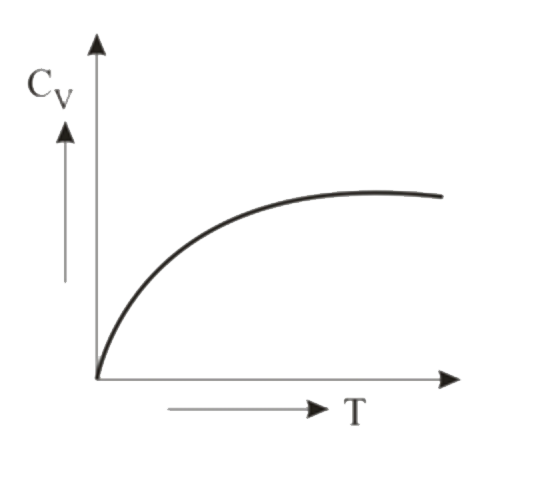
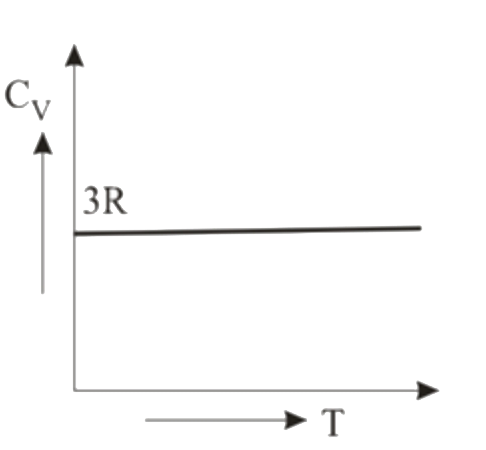
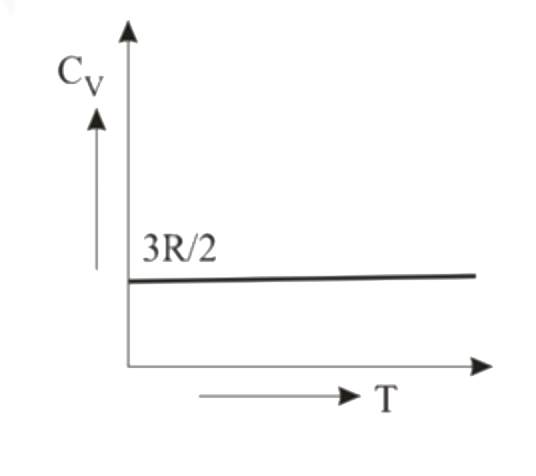
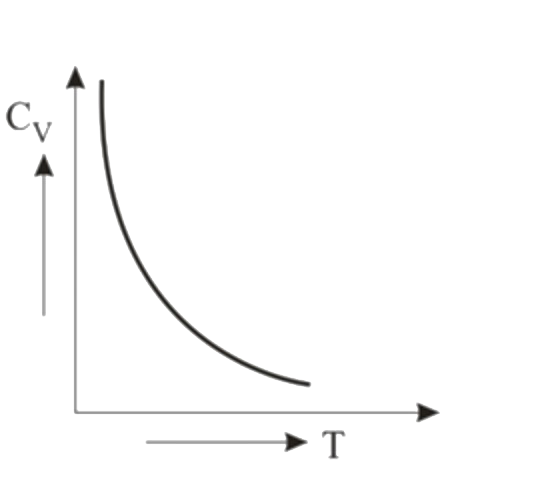
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Graph of specific heat at constant volume for a monatomic gas is
Text Solution
|
- Graph for specific heat at constant volume for a monoatomic gas
Text Solution
|
- A monatomic ideal gas is heated at constant volume until its pressure ...
Text Solution
|
- स्थिर आयतन पर किसी गैस की ग्राम-अणुक विशिष्ट ऊष्मा की परिभाषा लिखिये।
Text Solution
|
- Molar specific heat of a monatomic gas at constant pressure is
Text Solution
|
- Molar specific heat at constant volume Cv for a monatomic gas is
Text Solution
|
- स्थिर आयतन पर गैस की विशिष्ट ऊष्मा एवं स्थिर दाब पर गैस की विशिष्ट ऊष्...
Text Solution
|
- Graph for specific heat at constant volume for a monoatomic gas
Text Solution
|
- Two moles of a monatomic gas is mixed with three moles of a diatomic g...
Text Solution
|