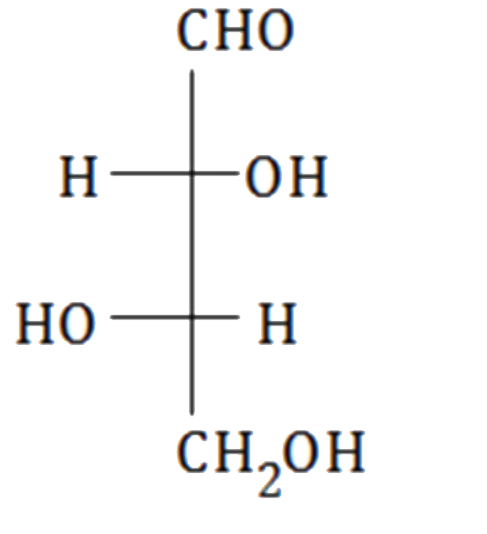
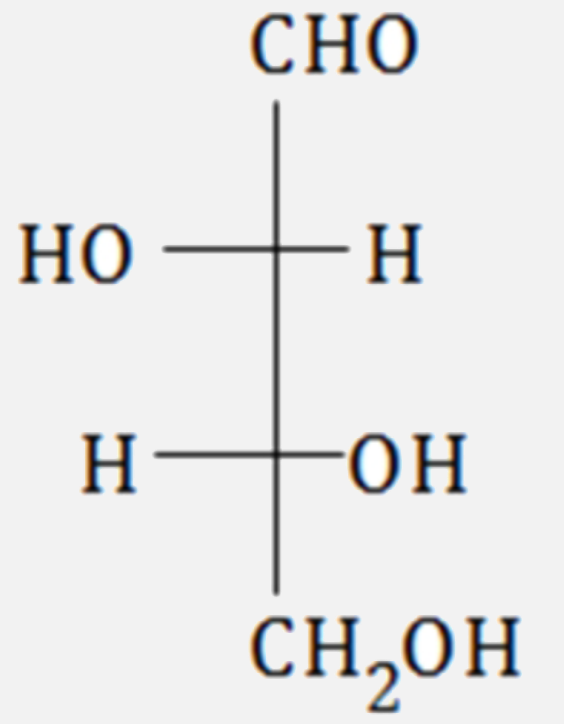
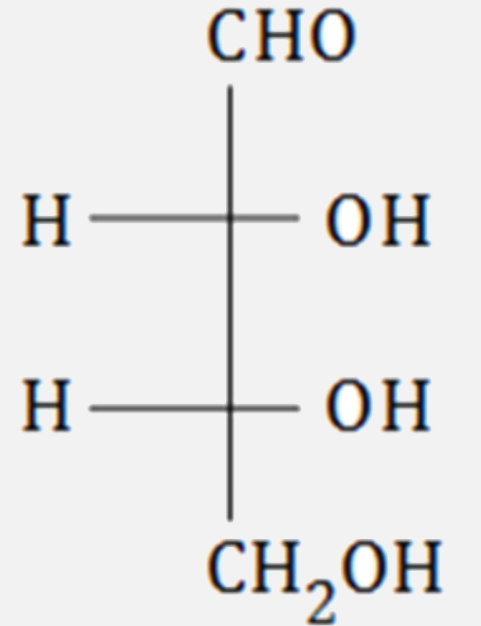
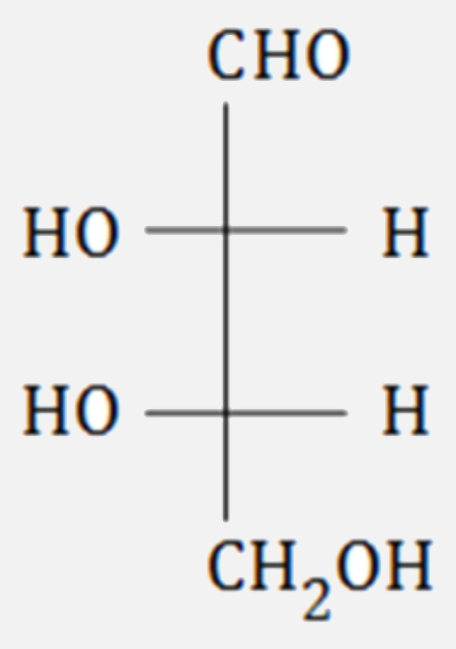
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following structures is a D-aldotetrose that gives a meso...
Text Solution
|
- Which of the following structure (s) is/are meso 2,3 butanediol?
Text Solution
|
- Which of the following structures repersent meso compound
Text Solution
|
- Which of the following structures is not meso 2,3-butanediol ?
Text Solution
|
- Toluene on oxidation with dilute HNO3 and alkaline KMnO4 gives
Text Solution
|
- Which of the following diacid readily gives anhybride on heating ?
Text Solution
|
- The reaction Ph-OH + dilute HNO3 to ? Gives predominately
Text Solution
|
- निम्न यौगिकों में से कौन सा ऑक्सीकरण पर कीटोन देता है?
Text Solution
|
- Which of the following structures is a D-aldotetrose that gives a meso...
Text Solution
|