A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
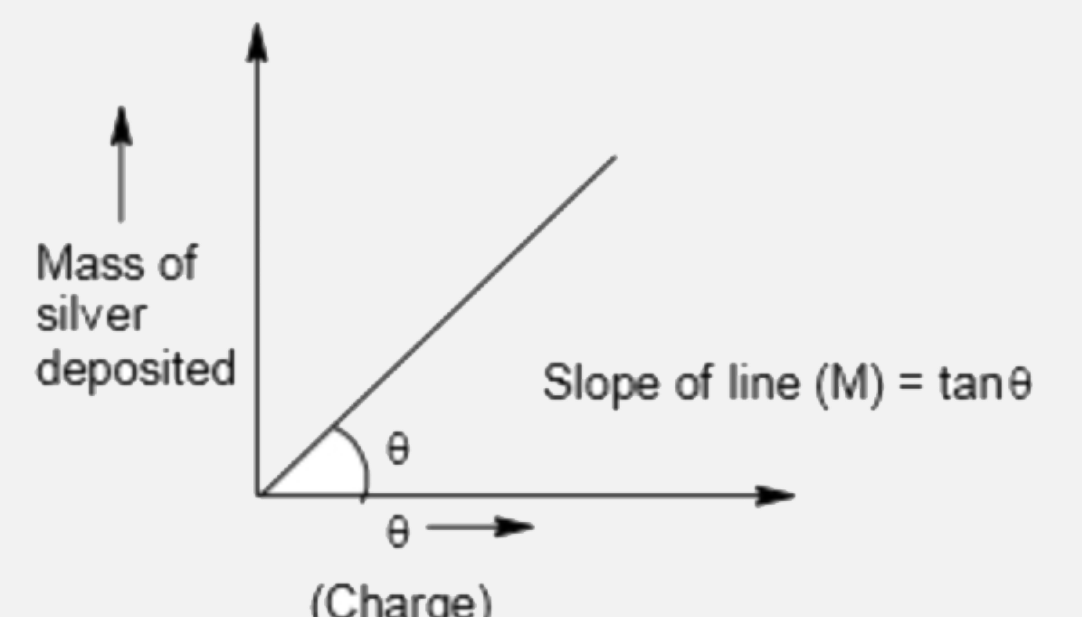
- Calculate the deposited mass of silver which is plotted against charge...
Text Solution
|
- If 0.5 amp current is passed through acidified silver nitrate soluti...
Text Solution
|
- A current o f1.5 A is passed through a silver voltameter for 35 minute...
Text Solution
|
- If 0.5 amp current is passed through acidified silver nitrate solution...
Text Solution
|
- Calculate the number of coulombs required to deposit 50 g of silver at...
Text Solution
|
- Calculate the mass of silver deposited from silver nitrate solution by...
Text Solution
|
- In the electrolysis of silver nitrate, the mass of silver deposited is...
Text Solution
|
- Calculate the deposited mass of silver which is plotted against charge...
Text Solution
|
- A solution of silver nitrate is electrolysed for 20 minutes with a cur...
Text Solution
|