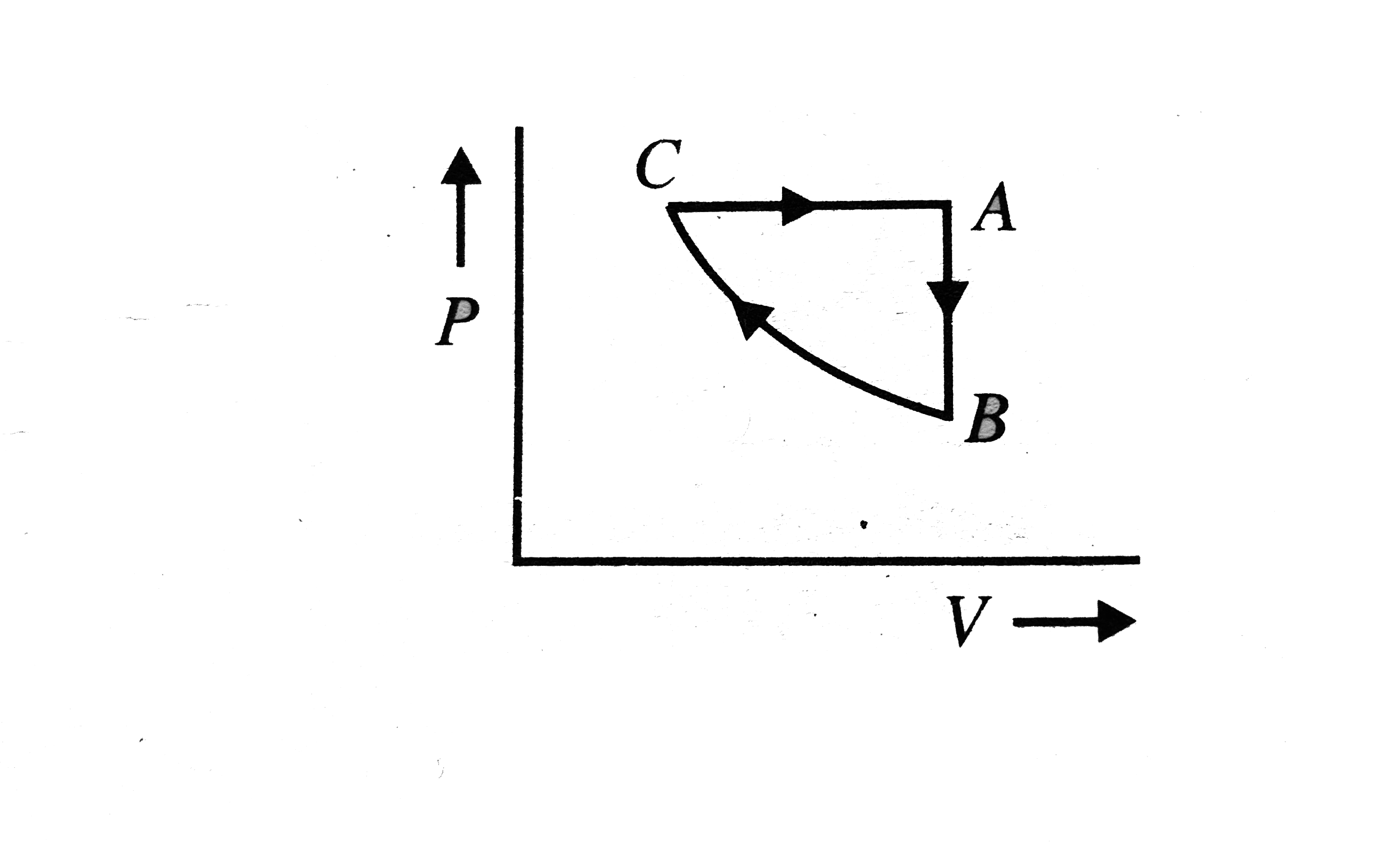A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A sample of an ideal gas is taken through the cyclic-process ABCA show...
Text Solution
|
- A sample of an ideal gas is taken through the cyclic process abca . It...
Text Solution
|
- A thermodynamic system is taken through the cycle abcda during the par...
Text Solution
|
- Consider the cyclic process ABCA, shown in, performed on a sample of ...
Text Solution
|
- A sample of an ideal gas is taken through the cyclic-process ABCA show...
Text Solution
|
- An ideal monoatomic gas undergoes a cyclic process ABCA as shown in th...
Text Solution
|
- A sample of an ideal gas in taken through the cyclic process abca in t...
Text Solution
|
- In figure, a sample of an ideal gas is taken through the cyclic proces...
Text Solution
|
- An ideal gas follows a cyclic process as shown in figure. Internal ene...
Text Solution
|