Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BODY BOOKS PUBLICATION-PEPIODIC TABLE AND ELECTRONIC CONFIGURATION-EXAMPLE
- Magnanese, a d-block elements exhibits different oxidation state.Why?
Text Solution
|
- Write the oxidation number and subshell electronic configuration K, CI...
Text Solution
|
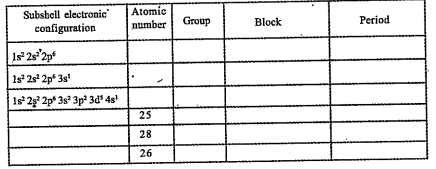
- Find out atomic number,group, block period using subshell electronic c...
Text Solution
|
- Write down the characteristics of s,d,p,f block elements.
Text Solution
|
- Write down subshell electronic configura-tion of Cu^(1+) and Cu^(2+)
Text Solution
|
- How many 's' subshell electrons are present in 1s^2, 2s^2, 2p^6, 3s^2,...
Text Solution
|
- 11,17,10 are the atomic number of elements X,Y and Z: Write down their...
Text Solution
|
- 11,17,10 are the atomic number of elements X,Y and Z: Write the molecu...
Text Solution
|
- 11,17,10 are the atomic number of elements X,Y and Z: Write down the o...
Text Solution
|
- Element 'X' is having atomic number 28, it gives two electrons to elem...
Text Solution
|
- Element 'X' is having atomic number 28, it gives two electrons to elem...
Text Solution
|
- Element 'X' is having atomic number 28, it gives two electrons to elem...
Text Solution
|
- Write down the group and period of each element.
Text Solution
|
- What are the use of writing electronic configuration this fashion?
Text Solution
|
- "24Cr-[Ar]3d^5 4s^1 Why chromium exhibits such electronic configurati...
Text Solution
|
- "29Cu-[Ar]3d^10 4s^1 Why chromium and copper exhibits such electronic ...
Text Solution
|
- The electronic configuration of the elements are given below. A-1s^2 ...
Text Solution
|
- The electronic configuration of the elements, are given below. A-1s^2...
Text Solution
|
- The electronic configuration of the elements are given below. A-1s^2 ...
Text Solution
|
- The electronic configuration of the eleme-nts, A,B,C,D are given below...
Text Solution
|