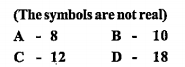Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BODY BOOKS PUBLICATION-PEPIODIC TABLE AND ELECTRONIC CONFIGURATION-EXAMPLE
- Two compounds XY2, XZ4 are given. The oxidation state of Z is 1. Write...
Text Solution
|
- Pick out the statements which suits to f block elements. a. All of the...
Text Solution
|
- The atomic number of four elements are given below. Write the sub-...
Text Solution
|
- The atomic number of four elements are given below. Which of them...
Text Solution
|
- The atomic number of four elements are given below. Write the chemi...
Text Solution
|
- The subshell electronic configuration of two elementsends asfollows.(S...
Text Solution
|
- The subshell electronic configuration of two elementsends asfollows.(S...
Text Solution
|
- The subshell electronic configuration of two elementsends asfollows.(S...
Text Solution
|
- Match the following.
Text Solution
|
- The atomic number of two elements are given below. Si-14 Ni-28: Write ...
Text Solution
|
- The atomic number of two elements are given below. Si-14 Ni-28: Find o...
Text Solution
|
- The element ‘X’ has 4 shells, and its3d sub-shell has6 electrons.(Symb...
Text Solution
|
- The element‘X’ has 4 shells, and its3d sub-shell has6 electrons.(Symbo...
Text Solution
|
- The element‘X’ has 4 shells, and its3d sub-shell has6 electrons.(Symbo...
Text Solution
|
- The element‘X’ has 4 shells, and its3d sub-shell has6 electrons.(Symbo...
Text Solution
|
- The outermost electronic configuration of the element A is 2s^2 2p^2. ...
Text Solution
|
- The outermost electronic configuration of the element A is 2s^2 2p^2. ...
Text Solution
|
- The outermost electronic configuration of the element A is 2s^2 2p^2. ...
Text Solution
|
- The figure of an incomplete periodic table is given below. : Which ...
Text Solution
|
- The figure of an incomplete periodic table is given below. Which o...
Text Solution
|