Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BODY BOOKS PUBLICATION-PEPIODIC TABLE AND ELECTRONIC CONFIGURATION-EXAMPLE
- The outermost electronic configuration of the element A is 2s^2 2p^2. ...
Text Solution
|
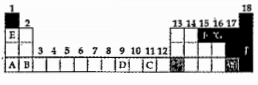
- The figure of an incomplete periodic table is given below. : Which ...
Text Solution
|
- The figure of an incomplete periodic table is given below. Which o...
Text Solution
|
- The figure of an incomplete periodic table is given below. Which el...
Text Solution
|
- The figure of an incomplete periodic table is given below. Which of...
Text Solution
|
- The figure of an incomplete periodic table is given below. Which of...
Text Solution
|
- Examine the given electronic configurations. A- 1s^2 2s^2 2p^6 3s^2 3p...
Text Solution
|
- Examine the given electronic configurations. A- 1s^2 2s^2 2p^6 3s^2 3...
Text Solution
|
- Examine the given electronic configurations. A-1s^2 2s^2 2p^6 3s^2 3p...
Text Solution
|
- Examine the given electronic configurations. A-1s^2 2s^2 2p^6 3s^2 3p...
Text Solution
|
- The atomic number of the elements X and Y are 20, 26 respectively. Whe...
Text Solution
|
- The atomic number of the elements X and Y are 20, 26 respectively. Whe...
Text Solution
|
- Arrange the following sub-shells in the in-creasing order of energy 5p...
Text Solution
|
- Last electron in f-block elements goes to: Which shell? Outer shell/Pe...
Text Solution
|
- Last electron in f-block elements goes to: Which sub-shell? Outer f-su...
Text Solution
|
- Sub-shell electronic configuration of X is given below.1s^2, 2s^2, 2p^...
Text Solution
|
- Sub-shell electronic configuration of X is given below.1s^2, 2s^2, 2p^...
Text Solution
|
- A compound of vanadium pentoxide (V2O5) is used as catalyst: What is t...
Text Solution
|
- A compound of vanadium pentoxide (V2O5) is used as catalyst: How vanad...
Text Solution
|
- A compound of vanadium pentoxide (V2O5) is used as catalyst: Write the...
Text Solution
|