Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BODY BOOKS PUBLICATION-REACTIVITY SERIES AND ELECTROCHEMISTRY-EXERCIES
- The picture of a Galvanic Cell given below. Write the chemical equ...
Text Solution
|
- Identify the relation and fill in the blanks. Electrolytic cell. Elect...
Text Solution
|
- Arragne the below given metals in the decreasing order of their reacti...
Text Solution
|
- Fill in the incomplete blanks.
Text Solution
|
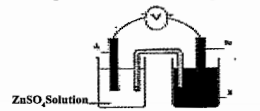 Write the chemical equation at the anode and cathode.
Write the chemical equation at the anode and cathode.