Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BODY BOOKS PUBLICATION-PRODUCTION OF METALS-EXERCISE
- Given below are the equations for the reactions taking place inside th...
Text Solution
|
- Fill in the blanks: Haematite-Iron. Bauxite-…………….
Text Solution
|
- Fill in the blanks: Sulphide ores-Froth Floatation. …………….-Leaching
Text Solution
|
- Identify the odd one out of the following. Find the reason also, Cryol...
Text Solution
|
- From those given below choose the metals that can be refined by liquat...
Text Solution
|
- Match the following:
Text Solution
|
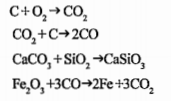 Reducing agent used in blast furnace.
Reducing agent used in blast furnace.