Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BODY BOOKS PUBLICATION-NOMENCLATURE OF ORGANIC COMPOUNDS AND ISOMERISM-EXERCISE
- Write the molecular formula.
Text Solution
|
- Identify the relation and complete: COOH-Acid, OH-…………….
Text Solution
|
- Compounds havign same molecular formula but different physical and che...
Text Solution
|
- Molecular formula of pentene is ………………..
Text Solution
|
- Observe the structure and answer the questions. CH3-underset(CH2-CH3)C...
Text Solution
|
- Observe the structure and answer the questions. CH3-underset(CH2-CH3)C...
Text Solution
|
- Observe the structure and answer the questions. CH3-underset(CH2-CH3)C...
Text Solution
|
- The molecular formula of a compound of C4H(10): What is the isomerism ...
Text Solution
|
- The molecular formula of a compound of C4H(10): Write 2 possible struc...
Text Solution
|
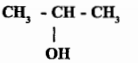 Write the molecular formula.
Write the molecular formula.