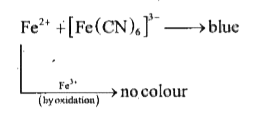A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE D- AND F-BLOCK ELEMENT
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|40 VideosTHE D- AND F-BLOCK ELEMENT
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|40 VideosSURFACE CHEMISTRY
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL - II) (ASSERTION REASON TYPE QUESTIONS) |6 VideosTHE D-AND F-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL-III)|50 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-THE D- AND F-BLOCK ELEMENT-LEVEL-II
- Which one of the following statements is not correct?
Text Solution
|
- Consider a titration of potassium dichromate solution with acidified M...
Text Solution
|
- K3 [Fe(CN)6] is used as an external indicator in the dichromate estim...
Text Solution
|
- Potassium permanganate acts as an oxidant in neutral, alkaline as well...
Text Solution
|
- Which of the following statements regarding basicity of lanthanide 3^+...
Text Solution
|
- The correct order of magnetic moments (spin only values in B.M.) among...
Text Solution
|
- Cerium (Z 58) is an important member of the lanthanoids. Which of the ...
Text Solution
|
- Ammonia forms the complexion [Cu(NH3)4]^(2+) with copper ions in alkal...
Text Solution
|
- Magnetic moment of Cr (Z-24), Mn^+(Z=25) and Fe^+ (Z=26) " are " x, y,...
Text Solution
|
- HCl is not used to make the medium acidic in oxidation reactions of KM...
Text Solution
|
- 'Which of the following statement is not correct?
Text Solution
|
- The number of moles of acidified KMnO4 required to convert one mole of...
Text Solution
|
- The correct order of ionic radii of Ce, La, Pm and Yb in+3 oxidation s...
Text Solution
|
- All Cu(II) halides are known except iodide. The reason for this is tha...
Text Solution
|
- When Hg2 Cl2, ionizes, the ions produced and the unpaired electrons pr...
Text Solution
|
- What is the effect of shaking dilute H2SO4, with a small quantity of a...
Text Solution
|
- Philosopher's wool when heated with Bao at 1100°C gives the compound:
Text Solution
|
- Which one of the following statements is correct?
Text Solution
|
- Number of electrons transferred in each case when KMnO4, acts as an ox...
Text Solution
|
- The atomic numbers of vanadium(V), chromium (Cr), manganese (Mn) and i...
Text Solution
|