Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-ALCOHOL, PHENOL & ETHER -LEVEL II (ASSERTION - REASON TYPE)
- Assertion: Reaction of alcohols with SOCl2 is catalysed by the presenc...
Text Solution
|
- Assertion: Solubility of n-alcohl in water decreases with increase in ...
Text Solution
|
- Assertion: The bond angle in alcohols is slightly less than the tetr...
Text Solution
|
- Assertion: Addition reaction of water to but-1-ene in acidic medium yi...
Text Solution
|
- Assertion : Carbon oxygen bond length of phenol is slightly less than ...
Text Solution
|
- Assertion : In alcohols, the boiling point decreases with decrease in...
Text Solution
|
- Assertion : Alcohols and water are weaker acids than phenols. Reas...
Text Solution
|
- Assertion : Bromination of phenol takes place even in the absence of...
Text Solution
|
- Assertion : The cleavage of C-O bond in ethers takes place under dra...
Text Solution
|
- Assertion: Dipolemoment ofphenol is smaller than that of methanol. R...
Text Solution
|
- Assertion: Neopentyl alcohol on treatment with HCl gives neopentyl chl...
Text Solution
|
- Assertion: Phenoxide ion on treatment with an active alkyl h...
Text Solution
|
- Assertion:Phenol forms 2,4,6-tribromophenol on treatment with Br2 in c...
Text Solution
|
- Assertion: Alcohols have higher boiling points than ethers of comparab...
Text Solution
|
- Assertion: Commercially carboxylic acids are reduced to alcohols by co...
Text Solution
|
- Assertion: Ethers are not prepared by the dehydration of secondary and...
Text Solution
|
- Assertion: CO2 is non polar, while ROR is polar in nature. Reason: ...
Text Solution
|
- Assertion: t-Butyl methyl ether is not prepared by the reaction of t-b...
Text Solution
|
- Assertion: Anisole undergoes electrophilic substitution at o-and p-pos...
Text Solution
|
- Assertion: Acid catalysed dehydration of tert-butanol proceeds faster ...
Text Solution
|
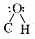 in alcohols is slightly less than the tetrahedral angle.
in alcohols is slightly less than the tetrahedral angle. 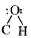 in alcohols is slightly less than the tetrahedral angle due to the repulsion between the unshared electronpairs of oxygen.
in alcohols is slightly less than the tetrahedral angle due to the repulsion between the unshared electronpairs of oxygen.