Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BODY BOOKS PUBLICATION-Chemical Kinetics-Example
- For the reaction A + BrarrC, itIs found that doubling the concentratio...
Text Solution
|
- In the Arrheniusequation,E represents
Text Solution
|
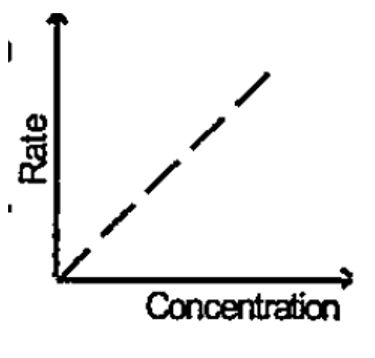
- Following graph is a plot of the rate of reaction concentration of the...
Text Solution
|
- Following graph is a plot . of the rate of reaction VS concentration o...
Text Solution
|
- Order of the photochemical reaction occurlng between Hydrogen and Chlo...
Text Solution
|
- Observe the relationship between the first two terms and fill in the b...
Text Solution
|
- Choose the correctly matched pair.
Text Solution
|
- Identify the order of a reaction if the units of its rate constant is...
Text Solution
|
- What Is the rate of disappearance of hydrogen in the reaction ?3H2+N2r...
Text Solution
|
- The temperature dependence ofis expressed by Arrhenius equation.
Text Solution
|
- The energy needed to form the intermediate called activated complex is...
Text Solution
|
- The number of collisions per second per unit volume of the reaction mi...
Text Solution
|
- Can we have reactions which proceed with constant rate?
Text Solution
|
- Instantaneous rate of reaction is preferred to average rate. Why?
Text Solution
|
- Identity the graph (A or B), represents the change in concentration of...
Text Solution
|
- In a class room discussion a student argues that average rate and inst...
Text Solution
|
- In a class room discussion a student argues that average rate and inst...
Text Solution
|
- From the rate expression for the following reaction determine the orde...
Text Solution
|
- If half-life of a reaction Is directly proportional to Initial concent...
Text Solution
|
- Give a relation which connects rate constant with temperature.
Text Solution
|