Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BODY BOOKS PUBLICATION-Chemical Kinetics-Example
- Can we have reactions which proceed with constant rate?
Text Solution
|
- Instantaneous rate of reaction is preferred to average rate. Why?
Text Solution
|
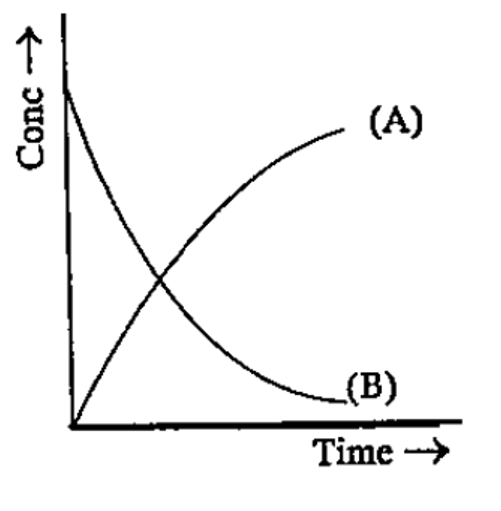
- Identity the graph (A or B), represents the change in concentration of...
Text Solution
|
- In a class room discussion a student argues that average rate and inst...
Text Solution
|
- In a class room discussion a student argues that average rate and inst...
Text Solution
|
- From the rate expression for the following reaction determine the orde...
Text Solution
|
- If half-life of a reaction Is directly proportional to Initial concent...
Text Solution
|
- Give a relation which connects rate constant with temperature.
Text Solution
|
- For first order reaction half-life period Is Independent of initial co...
Text Solution
|
- For first order reaction half-life period Is Independent of initial co...
Text Solution
|
- A first order reaction is 20% complete in 10 min. Calculate the spec...
Text Solution
|
- A first order reaction is 20% complete in ten minutes. Calculate time ...
Text Solution
|
- Mention the factors that affect the rate of a chemical reaction.
Text Solution
|
- The rate constant for a first order reaction is 60 (s)^(-1) .How much ...
Text Solution
|
- From the rate expression for the following reactions determine the ord...
Text Solution
|
- From the rate expression for the following reactions determine the ord...
Text Solution
|
- For a reaction, A + H2OrarrB, the rate law is rated [A]. What Is its m...
Text Solution
|
- 75% of a reaction of first order was completed in 64 minutes. When was...
Text Solution
|
- Rate of a reaction is the change in concentration of reactant or produ...
Text Solution
|
- For a reaction A + BrarrC + D. The order with respect to A is 1 and th...
Text Solution
|