Similar Questions
Explore conceptually related problems
Recommended Questions
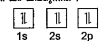
- Which rule is violated in the following orbital diagram?
Text Solution
|
- The orbital diagram in which both the Pauli's exlusion principle and H...
Text Solution
|
- The orbital diagram in which the Aufbau principle is violated
Text Solution
|
- The orbital diagram in which both Pauli's exclusion principle and Hund...
Text Solution
|
- The orbital diagram in which both the pauli's exclusion principal and ...
Text Solution
|
- In which of the following orbital diagram Aufbau principal is not viol...
Text Solution
|
- The orbital diagram in which both the Pauli's exclusion principle and ...
Text Solution
|
- The orbital diagram in which Hund's rule and Aufbau principle is viola...
Text Solution
|
- The orbital diagram in which both Pauli's exclusion principle and Hund...
Text Solution
|