Similar Questions
Explore conceptually related problems
Recommended Questions
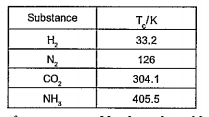
- Critical temperature of some substance are given below: It is not pos...
Text Solution
|
- At which of the following conditions can a gas be liquified? T(c) and ...
Text Solution
|
- Which one of the following is / true :- (i) At critical temp the sur...
Text Solution
|
- The critical temperatures of CO2 gas and N2 gas are 31^(@)C and -147^(...
Text Solution
|
- Why cannot CO(2) gas be liquified above 31.1^(@)C ?
Text Solution
|
- The pressure and temperature of a gas are P and T respectively. If the...
Text Solution
|
- Critical temperature (T(C)) and critical pressure (P(C)) of carbon dio...
Text Solution
|
- A real gas has critical temperature and critical pressure as 40^(@)C a...
Text Solution
|
- Consider following statements : (A): The gas whose critical tempera...
Text Solution
|