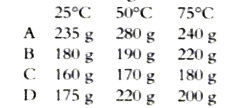A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
WATER, SOLUTION, SOLUBILITY AND HYDROGEN
PEARSON IIT JEE FOUNDATION|Exercise CONCEPT APPLICATION (LEVEL 2)|24 VideosWATER, SOLUTION, SOLUBILITY AND HYDROGEN
PEARSON IIT JEE FOUNDATION|Exercise CONCEPT APPLICATION (LEVEL 3)|10 VideosWATER, SOLUTION, SOLUBILITY AND HYDROGEN
PEARSON IIT JEE FOUNDATION|Exercise CONCEPT APPLICATION (LEVEL 1) |1 VideosSYNTHETIC FIBRES AND PLASTICS
PEARSON IIT JEE FOUNDATION|Exercise COMPETITION CORNER (Choose the correct option)|53 Videos
Similar Questions
Explore conceptually related problems
PEARSON IIT JEE FOUNDATION-WATER, SOLUTION, SOLUBILITY AND HYDROGEN-CONCEPT APPLICATION (LEVEL 1) (MULTIPLE CHOICE QUESTIONS)
- In the given graph, identify the substance associated with the highest...
Text Solution
|
- Reaction: Non-metal + H2 A A+PbOtoPb- Non-metal If A formed in th...
Text Solution
|
- Identify the binary solution among the following .
Text Solution
|
- Hydrogen acts as an oxidizing agent when it
Text Solution
|
- Tyndal effect cannot be shown by
Text Solution
|
- Sodium catches fire and burns with a
Text Solution
|
- Which of the following metals on reaction with steam provides a coatin...
Text Solution
|
- The brown coloured substance formed when steam is passed over red hot ...
Text Solution
|
- Which among the following metals react only with steam ?
Text Solution
|
- Crystals can be made
Text Solution
|
- Washing soda is an example of a/an substance .
Text Solution
|
- Which among the following substance acts as a desiccating agent ?
Text Solution
|
- Which of the following metals is unsuitable for the preparation of hyd...
Text Solution
|
- The reaction between perfectly dry hydrogen and chlorine take place in...
Text Solution
|
- Which of the following metals on treatment with concentrated alkali gi...
Text Solution
|
- Arrange the chemicals in sequence for the removal of impurities H2S, S...
Text Solution
|
- The specific heat capacity of water is
Text Solution
|
- Colour changes observed during the reaction between metal oxides and h...
Text Solution
|
- Amount of solutes A, B, C, D and E in 1500 g of water at 25°C, 50°C an...
Text Solution
|
- Among the following oxides which one is converted to metal by treating...
Text Solution
|