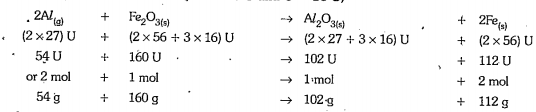Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BEYOND PUBLICATION-CHEMICAL EQUATIONS-EXERCISE
- Al(s) + Fe2O3(s) rarr Al2O3(s) + Fe(s) (atomic masses of Al = 27 U Fe ...
Text Solution
|
- Which of the following is correct ?
Text Solution
|
- Downward arrow in chemical equation indicates-
Text Solution
|
- The colour of the precipitate of lead iodide is-
Text Solution
|
- which of the following is a skeleton reaction?
Text Solution
|
- CaO reacts with water to form……….
Text Solution
|
- Ammonia is formed by the reaction of gases…………
Text Solution
|
- A solution of potassium iodide reacts with lead nitrate to give…..
Text Solution
|
- When a smal piece of zinc metal is added to a solution of Copper sulph...
Text Solution
|
- The chemical reaction in which energy is absorbed to form a new compou...
Text Solution
|
- The substances that are present on left side of a chemical equation ar...
Text Solution
|
- A chemical equation should be balanced because the law……. Should be v...
Text Solution
|
- C+O2toCO2+Q. This is reaction.
Text Solution
|
- Ca(OH)2+CO2toCaCO3+H2O. In this reaction shiny finish to walls is due ...
Text Solution
|
- Fe2O3 + 2Al -rarr Al2O3 + 2Fe. ఈ చర్య దేనికి ఉదాహరణ?
Text Solution
|
- Fill in the blanks: C6H12O6+6O2 to......+6H2O+Q
Text Solution
|
- Pb(NO3)2 +…………….. = PbI2+2KNO3
Text Solution
|
- NaCl + AgNO3rarr…………..+ NaNO3
Text Solution
|
- Fill in the blanks: Na2SO4+...... to BaSO4 +2NaCl
Text Solution
|
- Balance the following chemical equations. i) Zn((s))+AgNO(3(aq))rarr...
Text Solution
|
- If some amount of energy is released in a chemical reaction, then it i...
Text Solution
|