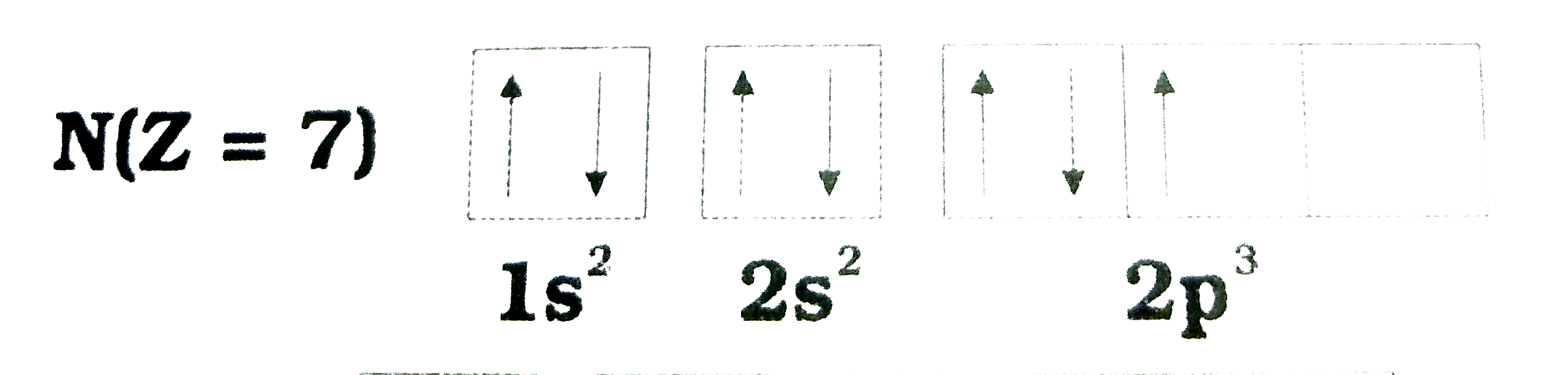Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BEYOND PUBLICATION-STRUCTURE OF ATOM-EXERCISE
- Following orbital diagram shows the electronic configuration of nitrog...
Text Solution
|
- An emission spectrum consists of bright spectral lines on a dark back ...
Text Solution
|
- The maximum number of electrons that can be accommodated in the L-shel...
Text Solution
|
- If l = 1 for an atom, then the number of orbitals in its sub-shell is
Text Solution
|
- The quantum number which explains about size and energy of the orbit o...
Text Solution
|
- Bohr's model can explain
Text Solution
|
- What is the use of quantum numbers ?What is electronic configuration ?
Text Solution
|
- What is Aufbau principle ?
Text Solution
|
- What information does the electronic configuration of an atom provide ...
Text Solution
|
- What is an orbital ?
Text Solution
|
- Which electron shell is at a higher energy level K or L ?
Text Solution
|
- Who introduced magnetic quantum number ?
Text Solution
|
- What is the shape of d-orbital ?
Text Solution
|
- 'l' ' value of d- orbital is
Text Solution
|
- 1s^2 2s^2 2p^1 is the electronic configuration of
Text Solution
|
- Which of the following elements have high atomic weight ?
Text Solution
|
- Who proposed the spectrum of hydrogen atom ?
Text Solution
|
- How many number of orbitals are there in the sub-shell of a magnetic q...
Text Solution
|
- The values of spin quantum numbers are (ms)
Text Solution
|
- Name the principle , which says an orbital can hold only 2 electrons a...
Text Solution
|
- What is the shape of p-orbital ?
Text Solution
|