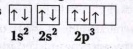
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BEYOND PUBLICATION-STRUCTURE OF ATOM-EXERCISE
- n = 2,l = 1, then the orbtial is represented as
Text Solution
|
- If two orbitals have’same (n + 1) value then
Text Solution
|
- The electronic configuration of nitrogen in denoted as This violates
Text Solution
|
- For an atom Sommerfeld picture is
Text Solution
|
- Which of the following is true according to quantum mechanical model o...
Text Solution
|
- Principal quantum number is related to
Text Solution
|
- Which of the following alloys contains Cu and Zn ?
Text Solution
|
- n and 'l' values for some orbitals are given. Which of the following o...
Text Solution
|
- Which of the following is violation of Pauli's exclusion principle ?
Text Solution
|
- When electric charge vibrates are produced.
Text Solution
|
- The range of wavelengths converging red colour to violet colour is cal...
Text Solution
|
- Select the correct answer. 'Electromagnetic energy can be gained or ...
Text Solution
|
- Which of the following is a failure of Bohr’s atomic model?
Text Solution
|
- Contribution of Summerfield to be model of atom
Text Solution
|
- The quantum number which explains about size and energy of the orbit o...
Text Solution
|
- If n=3, the main shell is
Text Solution
|
- If, n = 3, the maximum value of l is:
Text Solution
|
- The value of l for n = 2 are
Text Solution
|
- The orientation of orbital in space relative to the other orbitals in ...
Text Solution
|
- For a given value of ‘l’ the number of integer values of ml are
Text Solution
|