A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BEYOND PUBLICATION-CLASSIFICATION OF ELEMENTS THE PERIODIC TABLE-EXERCISE
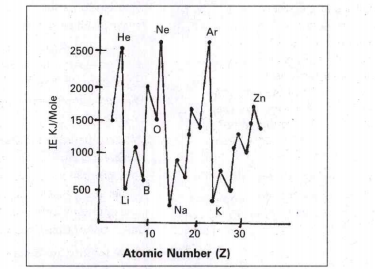
- lonization potential curve is the graph of atomic number versus ioniza...
Text Solution
|
- Number of elements present in period - 2 of the long form per...
Text Solution
|
- Nitrogen (Z=7) is the element of group V of the periodic table. Which...
Text Solution
|
- Electonic configuration of an atom is 2,8,7, . To which of the ...
Text Solution
|
- Which of the following is the most active metal ?
Text Solution
|
- Which of the following element is belongs to 3 period and also a 17 (V...
Text Solution
|
- Which of the following element belongs to the 4 period and IV B group ...
Text Solution
|
- Electron affinity values of halogens are measured in
Text Solution
|
- Which of the following elements are generally semi-conductors ?
Text Solution
|
- Which group elements are called halogen family ?
Text Solution
|
- In modern periodic table each periodic ends with
Text Solution
|
- Who noted that there were groups of elements with three elements known...
Text Solution
|
- Which of the following element family belongs to 16 (VIA) Group ?
Text Solution
|
- Which block elements are called the inner transition elements ?
Text Solution
|
- Which group elements are called Boron family ?
Text Solution
|
- Which of the following group elements are called alkali metal family ?
Text Solution
|
- Which block elements are called as transition metals ?
Text Solution
|
- According to which of the following characteristic of an element the m...
Text Solution
|
- Which of the following property is measured in 'pm' (pico meter) units...
Text Solution
|
- Which of the following group elements are nitrogen family ?
Text Solution
|
- By analysing which patterns the modern periodic table is proposed ?
Text Solution
|