A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SHARAM PUBLICATION-CHEMICAL KINETICS-EXERCISE
- For a reaction 1/2 A rarr 2B, the rate of disapperance of 'A' is relat...
Text Solution
|
- The rate of the reaction A rarr Products at the initial conc. Of 3.24...
Text Solution
|
- The concentration of R in the reaction R rarr P was measured on a func...
Text Solution
|
- For the reaction 2A + B rarr A2B, the rate law given is
Text Solution
|
- The half - life period of a first order reaction is 69.3 s.What is the...
Text Solution
|
- The reaction 2N2 O5 harr 2N2 O4 + O2 is
Text Solution
|
- For the following homogeneous reaction, the unit of rate constant is ...
Text Solution
|
- Which one of the following statement for the order of a reaction is in...
Text Solution
|
- A reaction involves two different reactants can never be
Text Solution
|
- t1/4 can be taken as time taken for the concentration of the reactant ...
Text Solution
|
- The rate of a chemical reaction doubles for every 10^@C rise of temper...
Text Solution
|
- The activation energy of exothermic reaction A rarr B is 80 KJ MOL^-1...
Text Solution
|
- The rate of reaction increases with temperature due to
Text Solution
|
- A chemical reaction proceeds following formula k = P.Z.e overset (-Ea ...
Text Solution
|
- The rate constant of a reaction is 2 xx 10^-2 lit mol^-1 sec^-1.What i...
Text Solution
|
- Out of zero , first and second order reaction which has the same unit ...
Text Solution
|
- Rate constant of which order reaction is independent of the concentrat...
Text Solution
|
- A reaction is 50 % complete in 2 hrs and 75% complete in 4 hrs what is...
Text Solution
|
- Under what condition does the rate of a reaction equal to rate constan...
Text Solution
|
- Rate constant of which order reaction is independent of the concentrat...
Text Solution
|
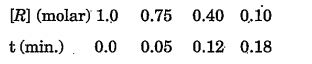 The order of the reaction is
The order of the reaction is