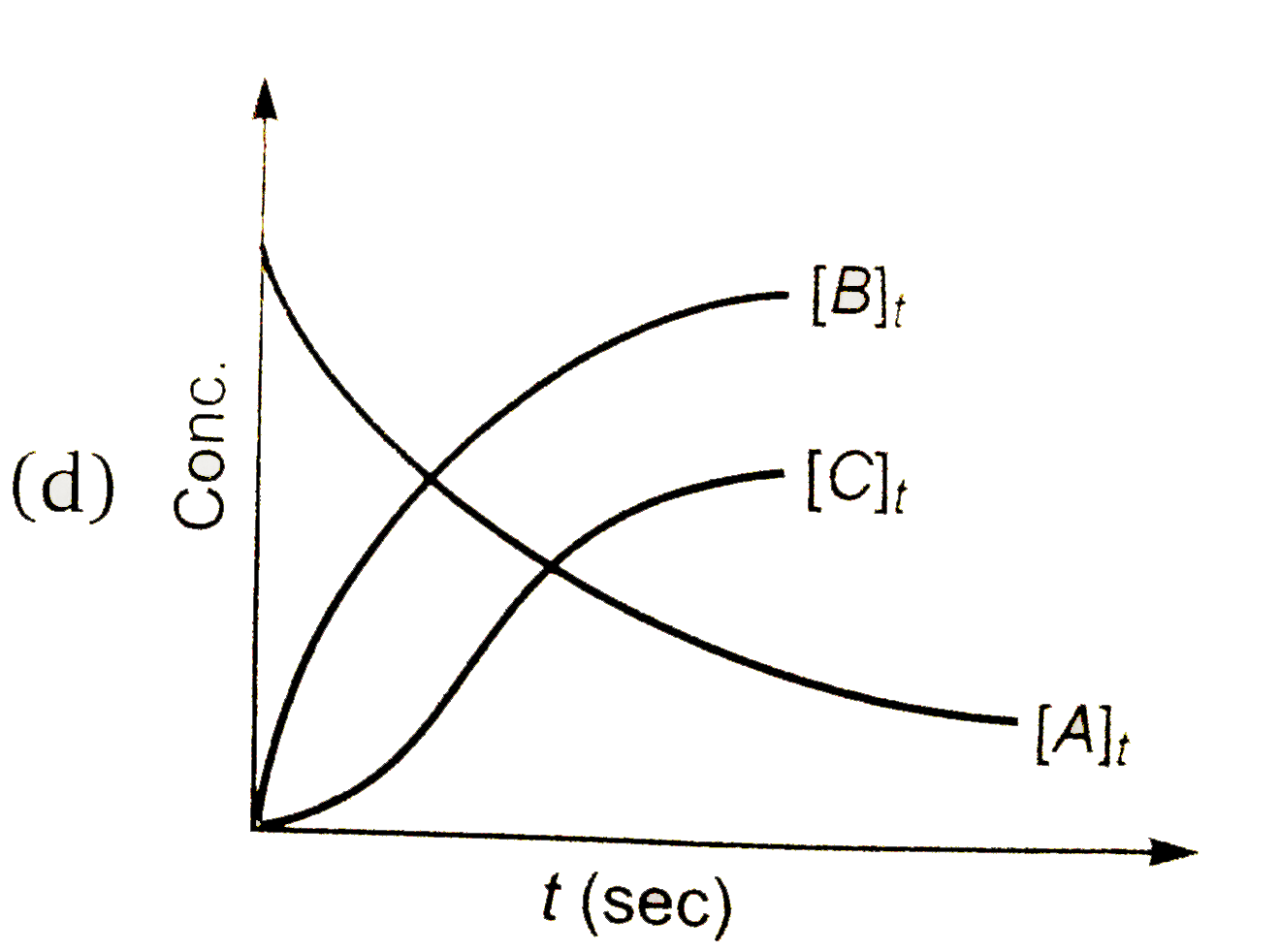
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETIC & NUCLEAR CHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - One Or More Answers Are Correct|11 VideosCHEMICAL KINETIC & NUCLEAR CHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Assertion - Reason Type Questions|3 VideosCHEMICAL KINETIC & NUCLEAR CHEMISTRY
NARENDRA AWASTHI|Exercise Level 2 (Q.33 To Q.35)|2 VideosSURFACE CHEMISTRY
NARENDRA AWASTHI|Exercise Level -2|1 Videos
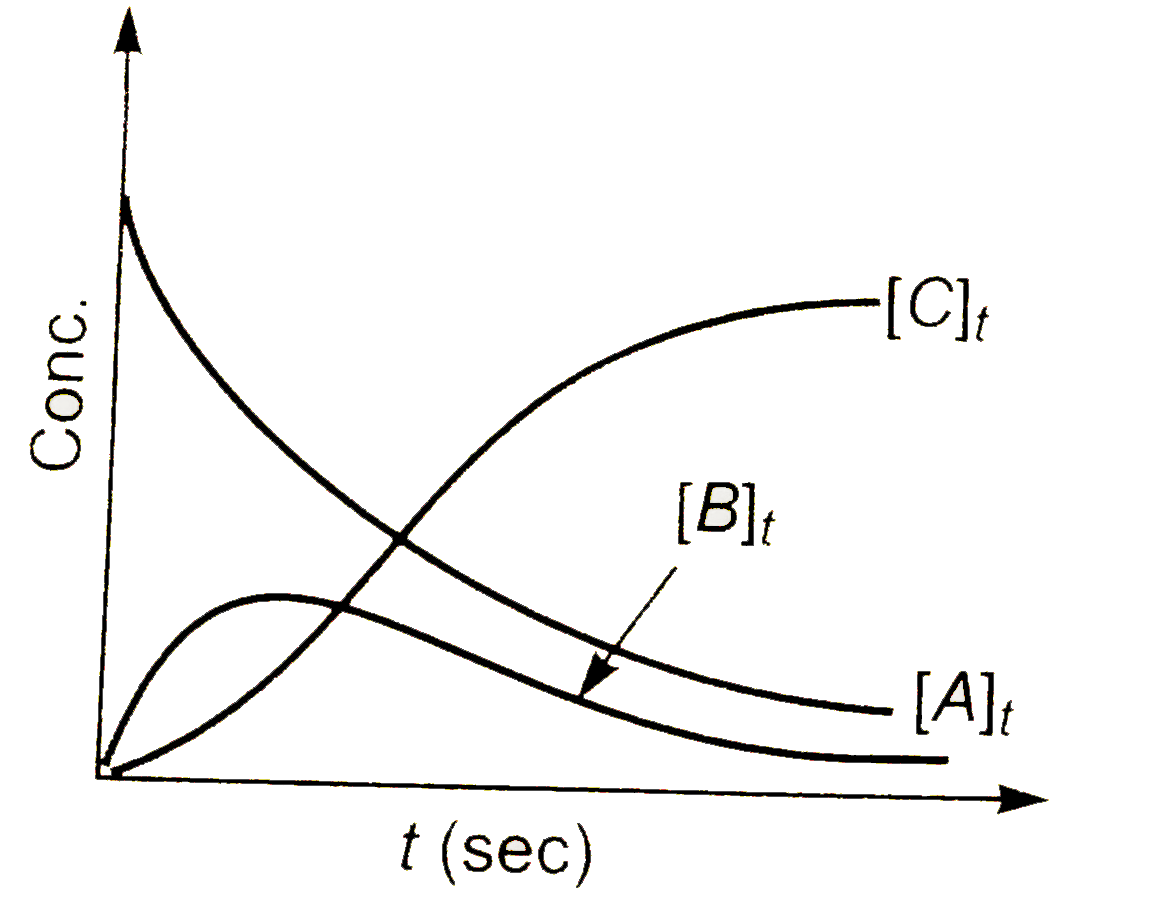 b)
b) 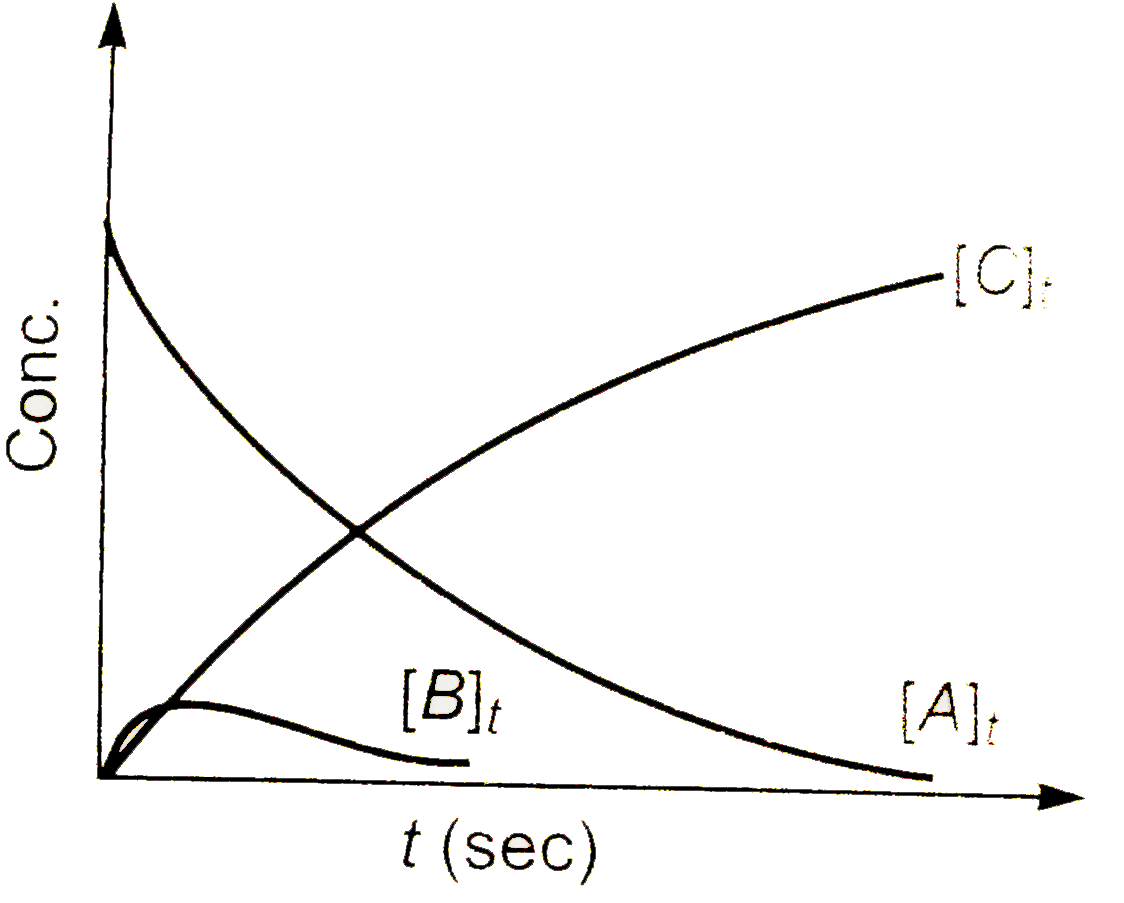
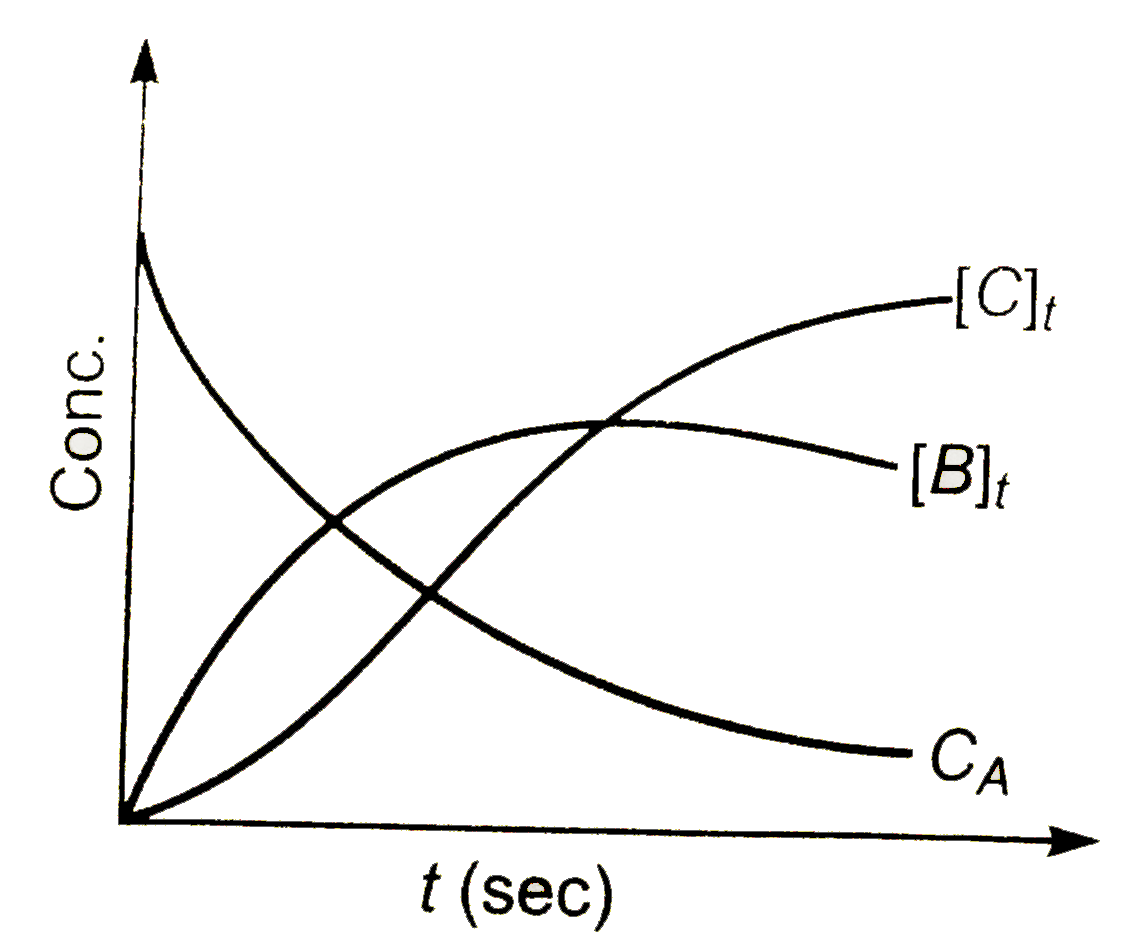 d)
d)