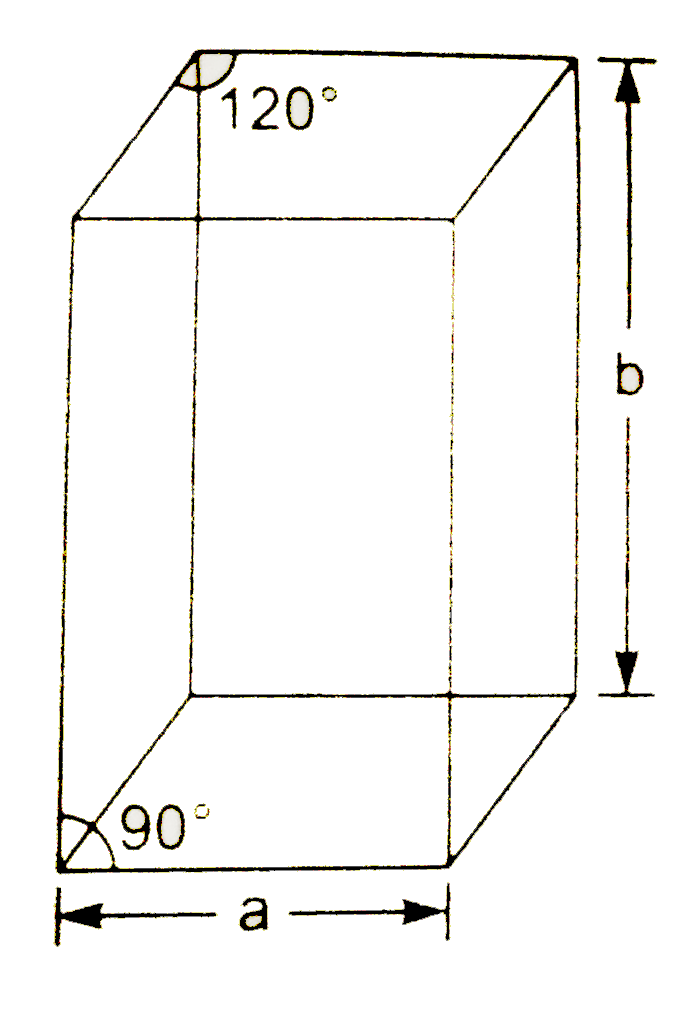
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
NARENDRA AWASTHI|Exercise Level 1 (Q.33 To Q.62)|1 VideosSOLID STATE
NARENDRA AWASTHI|Exercise Level 3 - One Or More Answers Are Correct|1 VideosIONIC EEQUILIBRIUM
NARENDRA AWASTHI|Exercise Assertin-Reason Type Questions|1 VideosSTOICHIOMETRY
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|20 Videos
Similar Questions
Explore conceptually related problems