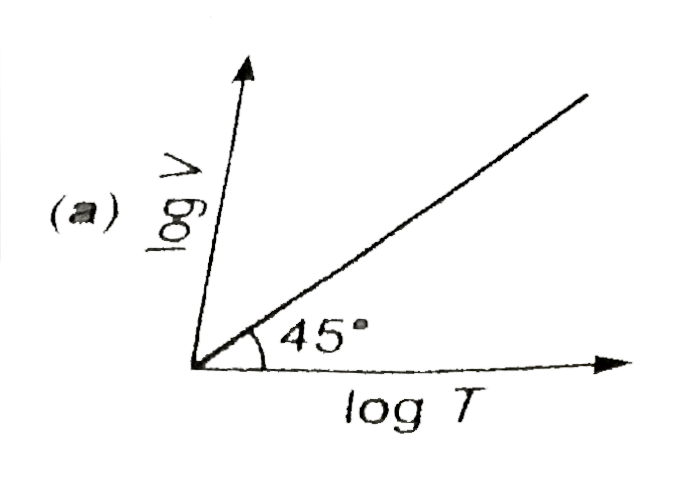
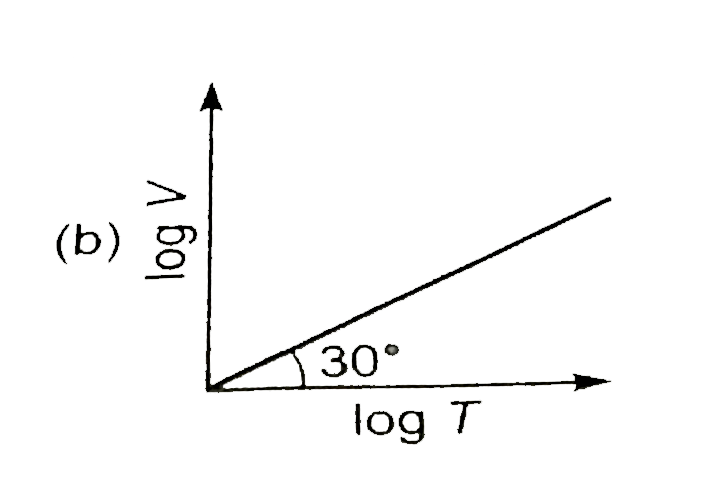
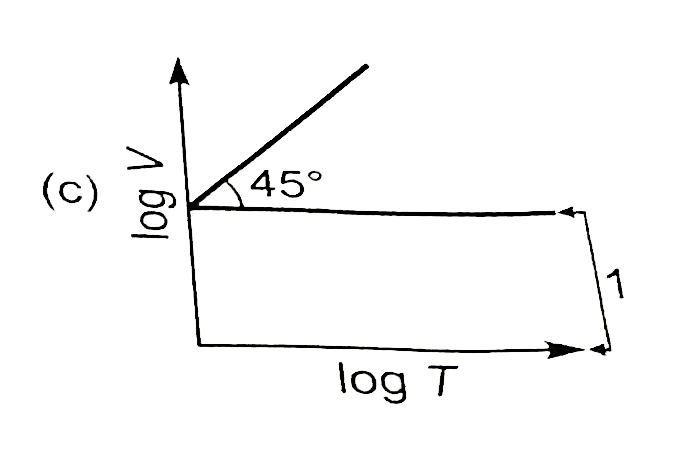
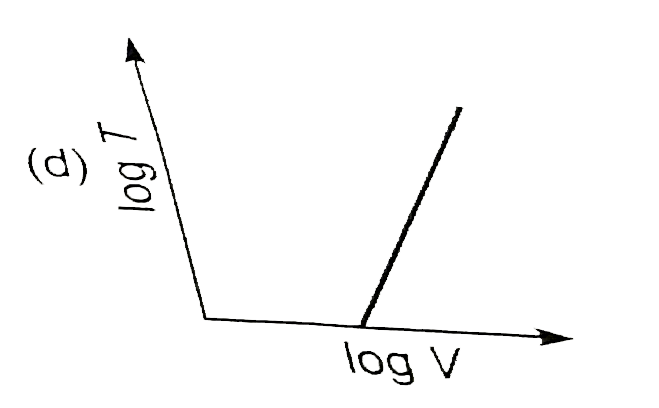
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 3 - One Or More Answers Are Correct|1 VideosTHERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 3 - Match The Column|2 VideosTHERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 2 (Q.1 To Q.30)|1 VideosSTOICHIOMETRY
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|20 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-THERMODYNAMICS-Exercise
- Which of the following expression is/are true ?
Text Solution
|
- If liquefied oxygen at 1 atmospheric pressure is heated from 50K to 30...
Text Solution
|
- For a closed container containing 100 mol of an ideal gas fitted with ...
Text Solution
|
- 10 mole of ideal gas expand isothermally and reversibly from a pressur...
Text Solution
|
- A heat engine carries one mole of an ideal monoatomic gas around the c...
Text Solution
|
- What is the final temperature of 0.10 mole monoatomic ideal gas that p...
Text Solution
|
- The work done by 1 mole of ideal gas during an adiabatic process is (a...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
- A gas expands adiabatically at constant pressure such that T propV^(-1...
Text Solution
|
- For a reversible adiabatic ideal gas expansion (dp)/(p) is equal to
Text Solution
|
- P-V plot for two gases (assuming ideal) during adiabatic processes are...
Text Solution
|
- Calculate the final temperature of a monoatomic idal gas that is compr...
Text Solution
|
- 5 mole of an ideal gas expand isothermally and irreversibly from a pre...
Text Solution
|
- With what minimum pressure (in kPa), a given volume of an ideal gas (C...
Text Solution
|
- The work done in adiabatic compression of 2 mole of an ideal monoatomi...
Text Solution
|
- One mole of an ideal gas (C(v,m)=(5)/(2)R) at 300 K and 5 atm is expan...
Text Solution
|
- 10 litre of a non linear polyatomic ideal gas at 127^(@)C and 2 atm pr...
Text Solution
|
- Calculate average molar heat capacity at constant volume of gaseous mi...
Text Solution
|
- 0.5 mole each of two ideal gasesA (C(v,m)=(5)/(2)R) and B (C(v,m)=3R) ...
Text Solution
|
- A cyclic process ABCD is shown in the P-V diagram. Which of the follow...
Text Solution
|