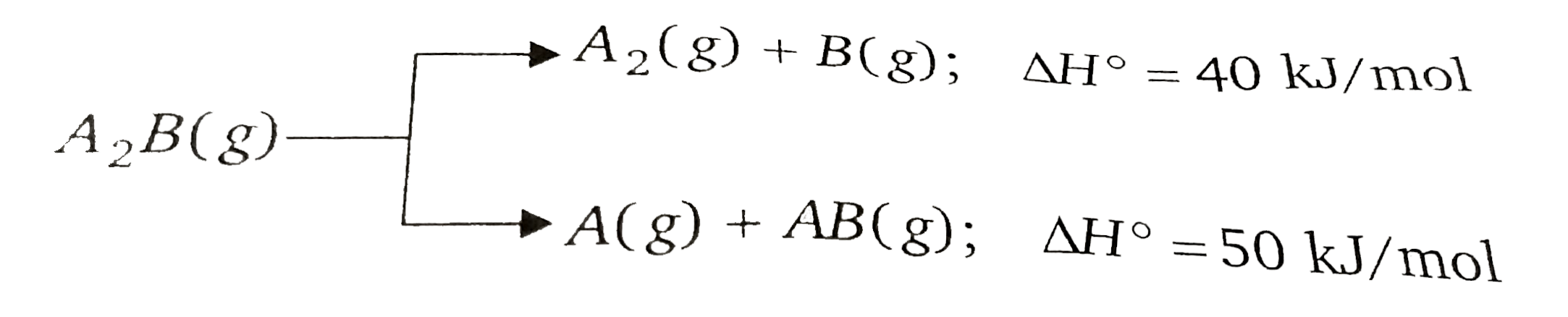A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 3 - One Or More Answers Are Correct|1 VideosTHERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 3 - Match The Column|2 VideosTHERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 2 (Q.1 To Q.30)|1 VideosSTOICHIOMETRY
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|20 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-THERMODYNAMICS-Exercise
- Which of the following equations are exothermic or endothermic? N2(g)...
Text Solution
|
- Which of the following is not correct? Robert Brown discovered the c...
Text Solution
|
- Substance A(2)B(g) can undergoes decomposition to form two set of prod...
Text Solution
|
- Write structure of diphenylmethane.
Text Solution
|
- The enthalpy change for the following reaction is 368 kJ. Calculate th...
Text Solution
|
- Calculate the enthalpy change for the reaction H(2)(g) + Cl(2)(g) to ...
Text Solution
|
- The table given below lists the bond dissociation energy (E("diss")) f...
Text Solution
|
- Write iupac names of the following: CH2=CH-CH(CH3) -CH=CH-CH=CH2
Text Solution
|
- Write iupac names of the following: CH=C-CH(CH3)-CH=CH2
Text Solution
|
- Write iupac names of the following: CH3-C=C-CH(CH3)2
Text Solution
|
- A heating coil is immersed in a 100 g sample of H(2)O (l) at a 1 atm ...
Text Solution
|
- A rigid and insulated tank of 3m^(3) volume is divided into two compar...
Text Solution
|
- Write iupac names of the following: Cl2-CH-CH2-OH
Text Solution
|
- For an ideal gas (C(p,m))/(C(v,m))=gamma. The molecular mass of the ga...
Text Solution
|
- 1 mole of an ideal gas A(C(v.m)=3R) and 2 mole of an ideal gas B are...
Text Solution
|
- Calculate the work done by the system in an irreversible (single step)...
Text Solution
|
- Write iupac names of the following: Br-CH2-CH2-CHO
Text Solution
|
- A gas (C(v.m) = (5)/(2)R) behaving ideally is allowed to expand revers...
Text Solution
|
- Two mole of an ideal gas is heated at constant pressure of one atmosp...
Text Solution
|
- 10 mole of an ideal gas is heated at constant pressure of one atmosphe...
Text Solution
|