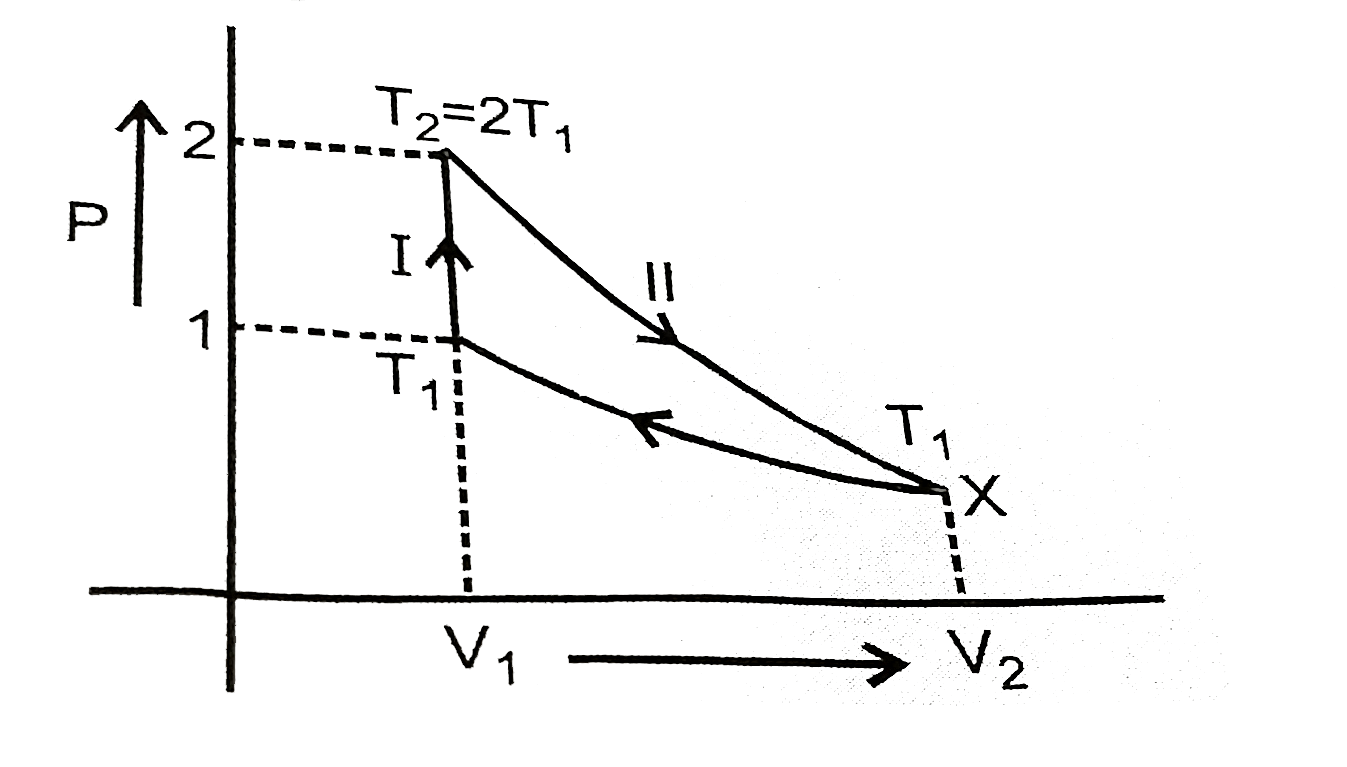
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 3 - One Or More Answers Are Correct|1 VideosTHERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 3 - Match The Column|2 VideosTHERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 2 (Q.1 To Q.30)|1 VideosSTOICHIOMETRY
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|20 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-THERMODYNAMICS-Exercise
- Indicate the sigma and pi bond in the following molecules : CH2=C=CH2
Text Solution
|
- Indicate the sigma and pi bond in the following molecules : CH3NH2
Text Solution
|
- Indicate the sigma and pi bond in the following molecules : HCONHCH3
Text Solution
|
- Write bond line formula for ethylene glycol
Text Solution
|
- Write bond line formula for 2-methylbutanal
Text Solution
|
- Write bond line formula for heptan-3-one
Text Solution
|
- The first law of thermodynamics for a closed system is dU = dq + dw, w...
Text Solution
|
- Write iupac name of CH3-CH2-CH2-C6H5
Text Solution
|
- Write iupac name of CH3-CH(Cl )-CH3
Text Solution
|
- Write iupac name of C6H5-CH2-C6H5
Text Solution
|
- If the boundary of system moves by an infinitesimal amount, the work i...
Text Solution
|
- Give the iupac names of the following compound : Cl-CH2-CH2-CHO
Text Solution
|
- Standard Gibb's energy of reaction (Delta(r )G^(@)) at a certain temp...
Text Solution
|
- Standard Gibb's energy of reaction (Delta(r )G^(@)) at a certain temp...
Text Solution
|
- Standard Gibb's energy of reaction (Delta(r )G^(@)) at a certain temp...
Text Solution
|
- Write iupac name of CH3-CH2-COOH
Text Solution
|
- Standard Gibb's energy of reaction (Delta(r )G^(@)) at a certain temp...
Text Solution
|
- Enthalpy of neutralzation is defined as the enthalpy change when 1 mol...
Text Solution
|
- Enthalpy of neutralzation is defined as the enthalpy change when 1 mol...
Text Solution
|
- Enthalpy of neutralzation is defined as the enthalpy change when 1 mol...
Text Solution
|