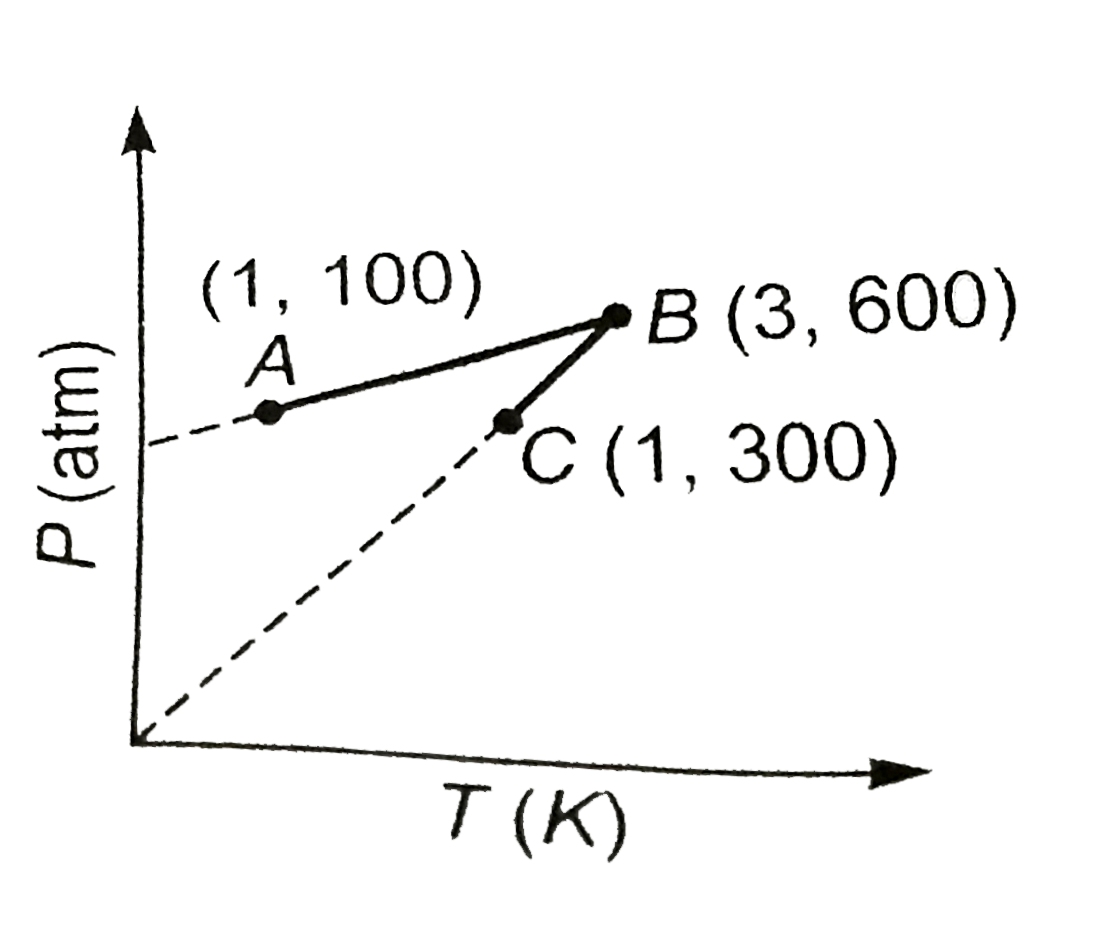A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 3 - One Or More Answers Are Correct|1 VideosTHERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 3 - Match The Column|2 VideosTHERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 2 (Q.1 To Q.30)|1 VideosSTOICHIOMETRY
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|20 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-THERMODYNAMICS-Exercise
- An adiabatic process is that process in which :
Text Solution
|
- The density of the ideal gas is given by
Text Solution
|
- One mole of an ideal gas is subjected to a two step reversible process...
Text Solution
|
- Which of the following is/are correct?
Text Solution
|
- DeltaHltDeltaU for the reaction(s) :
Text Solution
|
- Which of the following conditions will always lead to a non-spontaneou...
Text Solution
|
- For a process to be spontaneous at constant T and P :
Text Solution
|
- Write iupac name of CH3-CH(OH)-CH2OH
Text Solution
|
- Give the iupac names of the following compound : CH3-CH2-CH(CH3)-CH2-C...
Text Solution
|
- Give the iupac names of the following compound : CH3-CH(CH3)-CH2-CH2-C...
Text Solution
|
- Give the iupac names of the following compound : CH3-CH2-C(Cl)(Br)-CH2...
Text Solution
|
- Let f:R rarr R be a function defined by f(x+1)=(f(x)-5)/(f(x)-3), fora...
Text Solution
|
- Match the following : .
Text Solution
|
- Select correct statement(s)
Text Solution
|
- For an isolated system, the entropy :
Text Solution
|
- The normal boiling point of a liquid X is 400 K. DeltaH("vap") at norm...
Text Solution
|
- Select correct statement(s)
Text Solution
|
- Select correct statement(s) for the reaction H(2)O(g)+CO(g)rarrH(2)(g)...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|