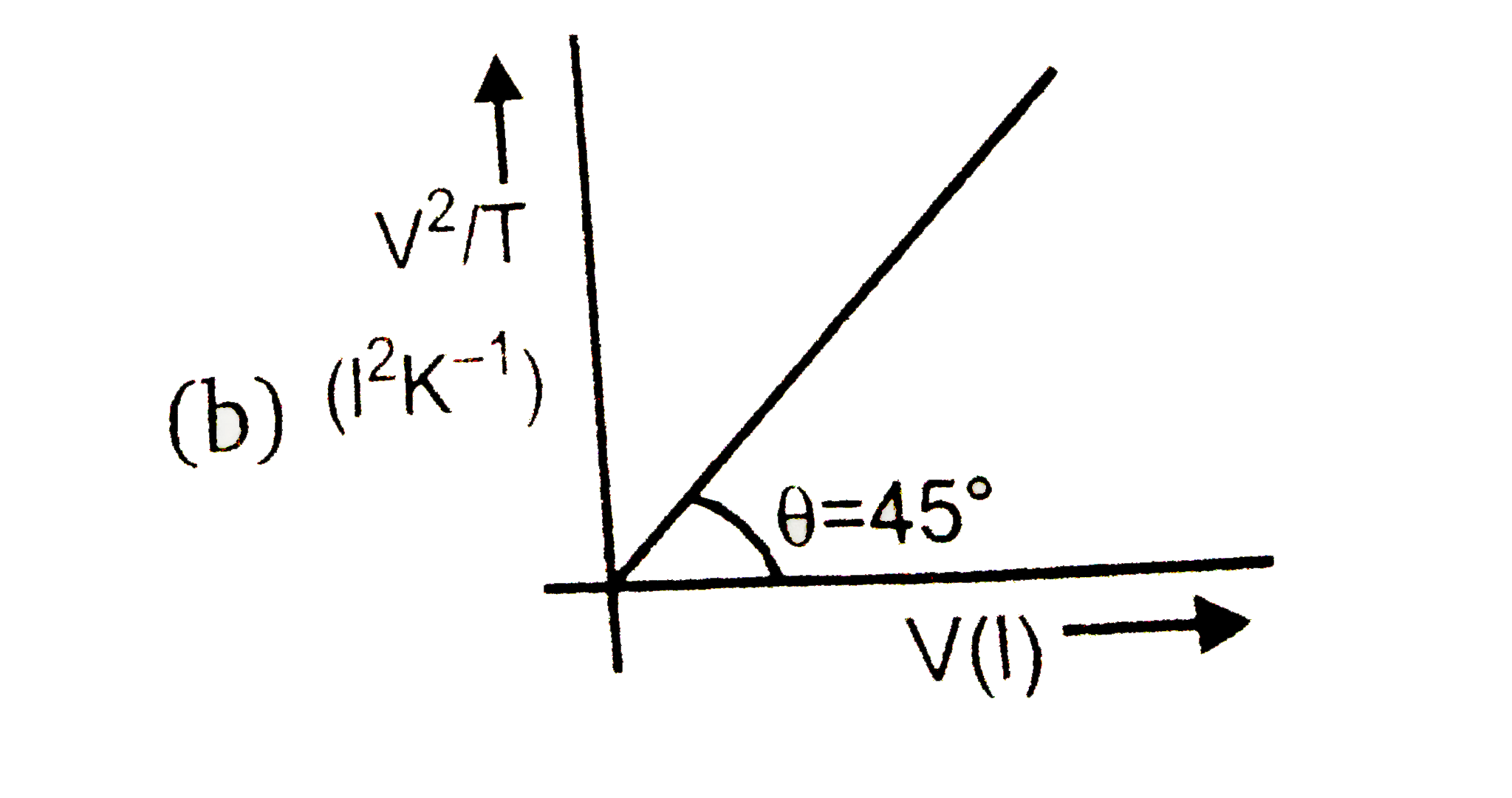
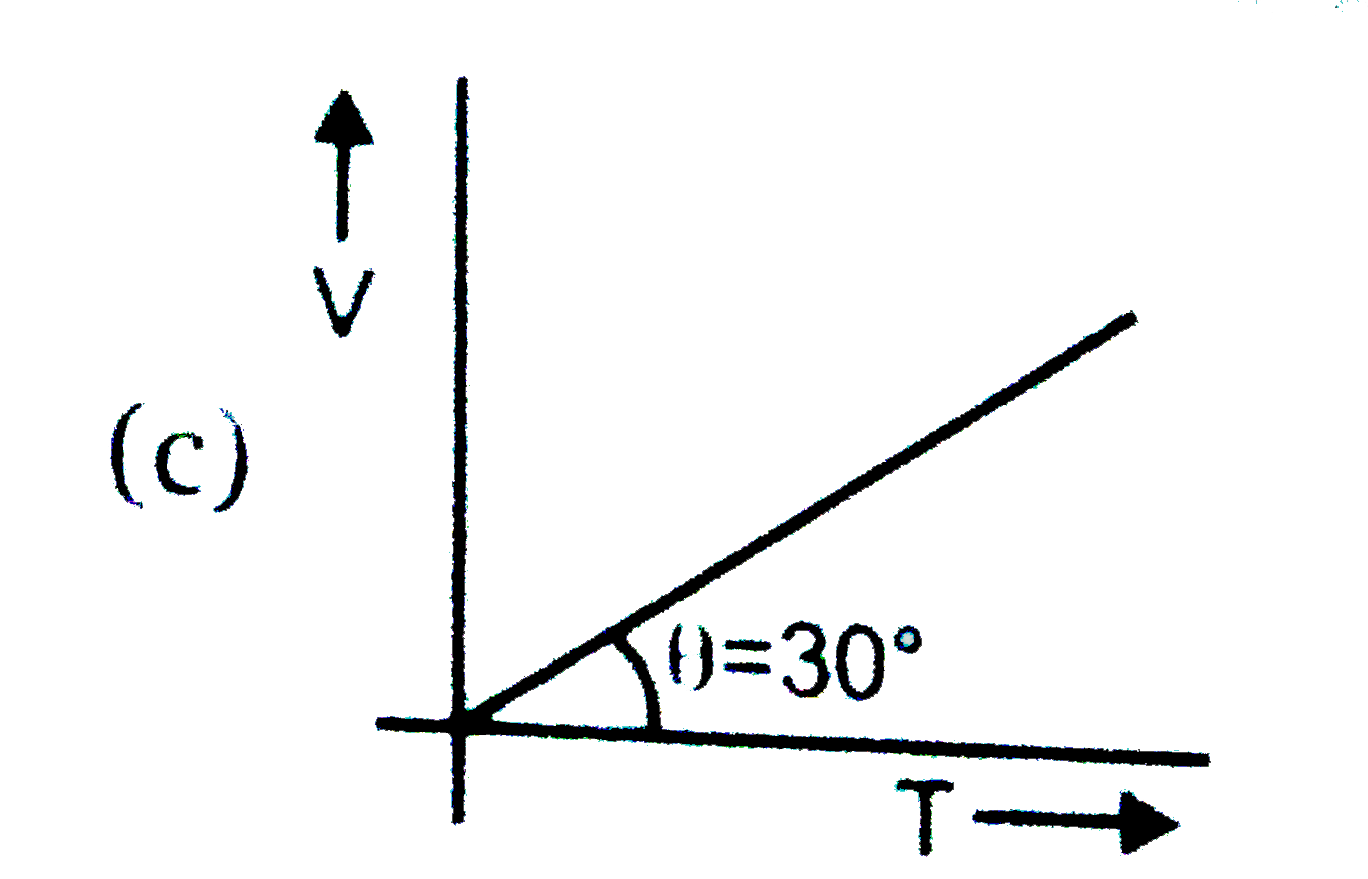
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE
NARENDRA AWASTHI|Exercise Level 1 (Q.151 To Q.176)|1 VideosGASEOUS STATE
NARENDRA AWASTHI|Exercise Level 2|1 VideosGASEOUS STATE
NARENDRA AWASTHI|Exercise Level 1 (Q.121 To Q.150)|1 VideosELECTROCHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|1 VideosIONIC EEQUILIBRIUM
NARENDRA AWASTHI|Exercise Assertin-Reason Type Questions|1 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-GASEOUS STATE-Exercise
- Among the following curves, which is not according to Charle's law ?
Text Solution
|
- Initial temperature of an ideal gas is 75^(@)C. At what temperature, ...
Text Solution
|
- Which is correct curve for Charle's law, when the curve is plotted at ...
Text Solution
|
- The temperature of an ideal gas increases in an:
Text Solution
|
- Which of the following curve does not represent Gay-Iusacc's law?
Text Solution
|
- Three flasks of equal volumes contain CH(4),CO(2), " and "Cl(2) gases ...
Text Solution
|
- Write the formula and identify the functional group , if any : N-methy...
Text Solution
|
- Equal volumes of gases at the same temperature and pressure contain eq...
Text Solution
|
- A 2.24L cylinder of oxygen at 1 atm and 273 K is found to develop a le...
Text Solution
|
- Which of the following curve is correct for an ideal gas ?
Text Solution
|
- The value of universal gas constant R depends on :
Text Solution
|
- At 0^(@)C and one atm pressure, a gas occupies 100 cc. If the pressure...
Text Solution
|
- 10 g of a gas at 1 atm and 273 K occupies 5 litres. The temperature at...
Text Solution
|
- Which of the following curve does not represent Gay-Iusacc's law?
Text Solution
|
- Densities of two gases are in the ratio 1: 2 and their temperatures ar...
Text Solution
|
- Two separate bulbs contain ideal gas A and B. The density of a gas A i...
Text Solution
|
- Volume of the air that will be expelled from a vessel of 300 cm^(3) wh...
Text Solution
|
- For an ideal gas V - T curves at constant pressure P(1) & P(2) are sho...
Text Solution
|
- Two flasks A and B have equal volumes. A is maintained at 300 K and B ...
Text Solution
|
- 2.8 g of a gas at 1atm and 273K occupies a volume of 2.24 litres. The ...
Text Solution
|