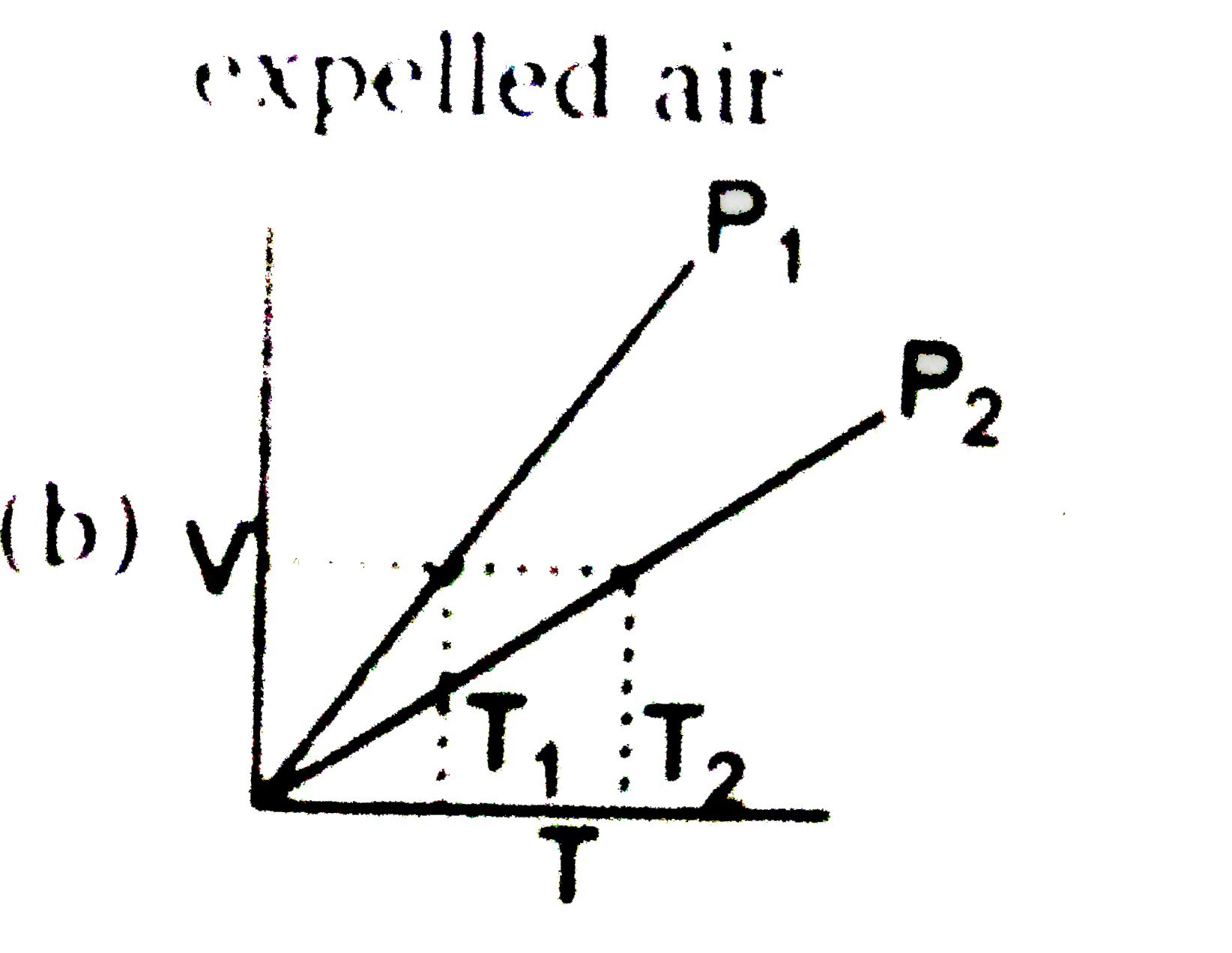A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE
NARENDRA AWASTHI|Exercise Level 1 (Q.151 To Q.176)|1 VideosGASEOUS STATE
NARENDRA AWASTHI|Exercise Level 2|1 VideosGASEOUS STATE
NARENDRA AWASTHI|Exercise Level 1 (Q.121 To Q.150)|1 VideosELECTROCHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|1 VideosIONIC EEQUILIBRIUM
NARENDRA AWASTHI|Exercise Assertin-Reason Type Questions|1 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-GASEOUS STATE-Exercise
- Two separate bulbs contain ideal gas A and B. The density of a gas A i...
Text Solution
|
- Volume of the air that will be expelled from a vessel of 300 cm^(3) wh...
Text Solution
|
- For an ideal gas V - T curves at constant pressure P(1) & P(2) are sho...
Text Solution
|
- Two flasks A and B have equal volumes. A is maintained at 300 K and B ...
Text Solution
|
- 2.8 g of a gas at 1atm and 273K occupies a volume of 2.24 litres. The ...
Text Solution
|
- Five grams each of the following gases at 87^(@)C and 750 mm pressure ...
Text Solution
|
- At what pressure a quantity of gas will occupy a volume of 60 mL, if i...
Text Solution
|
- 20 g of ideal gas contans only atoms of S and O occupies 5.6 L at...
Text Solution
|
- A small bubble rises from the bottom of a lake, where the temperature ...
Text Solution
|
- Argon is an inert gas used in light bulbs to retard the vaporization ...
Text Solution
|
- Calculate the volue of O(2) at 1 atm and 273 K required for the comple...
Text Solution
|
- The density of O(2)"(g)" is maximum at :
Text Solution
|
- At 27^(@)C a sample of ammonia gas exerts a pressure of 5.3 atm. What ...
Text Solution
|
- A certen amount of gas at 2.5^(@)C and at a pressure of 0.80 atm is ke...
Text Solution
|
- Which one of these graphs for an ideal gas havinga fixed amount, the a...
Text Solution
|
- The pressure of sodium vapour in a 1.0 L container is 10 torr at 1000^...
Text Solution
|
- An ideal gaseou smixture of enthance (C(2)H(6)) and enthene (C(2)H(4)...
Text Solution
|
- Give reason : a gas exerts pressure on the walls of the container.
Text Solution
|
- Air entering the lungs ends up in tiny sacs called alveoli.From the al...
Text Solution
|
- Starting out on a trip into the mountains, you inflate the tires on yo...
Text Solution
|
_E01_061_Q01.png)