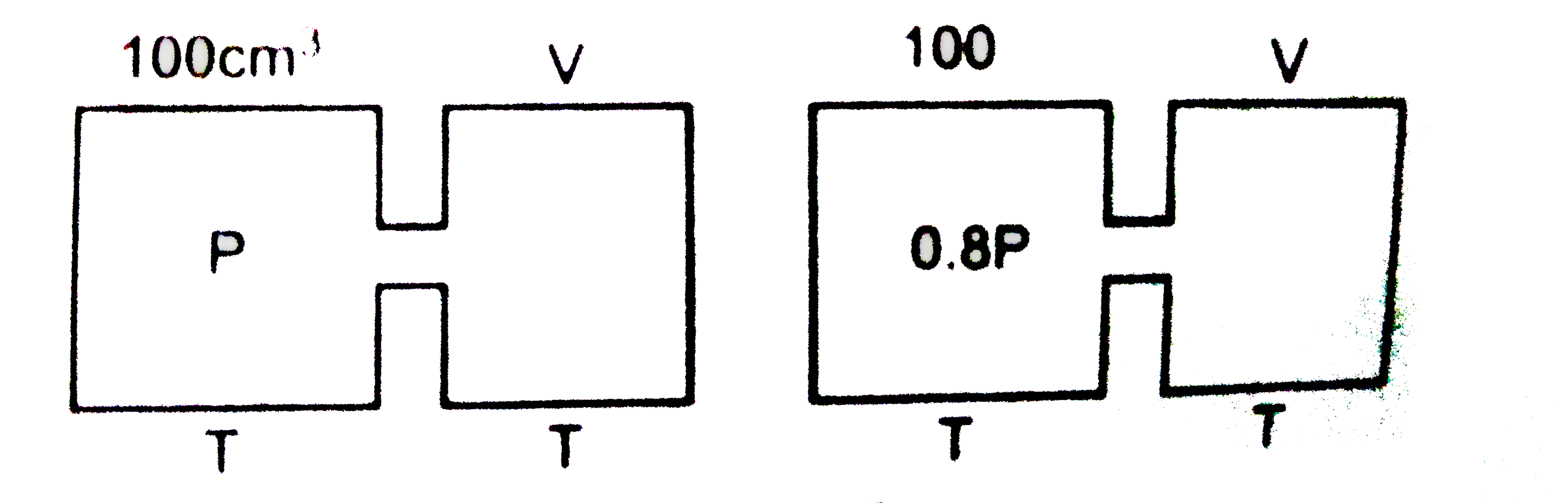Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE
NARENDRA AWASTHI|Exercise Level 1 (Q.151 To Q.176)|1 VideosGASEOUS STATE
NARENDRA AWASTHI|Exercise Level 2|1 VideosGASEOUS STATE
NARENDRA AWASTHI|Exercise Level 1 (Q.121 To Q.150)|1 VideosELECTROCHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|1 VideosIONIC EEQUILIBRIUM
NARENDRA AWASTHI|Exercise Assertin-Reason Type Questions|1 Videos
NARENDRA AWASTHI-GASEOUS STATE-Exercise
- Give the iupac name of : CH3-CH2-CH2-C (CH2-CH3)=CH2
Text Solution
|
- Give structure of cyclohexyl benzene
Text Solution
|
- The following organic compound are popularly known by their common nam...
Text Solution
|
- The following organic compound are popularly known by their common nam...
Text Solution
|
- The following organic compound are popularly known by their common nam...
Text Solution
|
- The following organic compound are popularly known by their common nam...
Text Solution
|
- The following organic compound are popularly known by their common nam...
Text Solution
|
- The following organic compound are popularly known by their common nam...
Text Solution
|
- The following organic compound are popularly known by their common nam...
Text Solution
|
- The vapour pressure of water at 80^(@)C is 355 mm of Hg. 1 L vessel co...
Text Solution
|
- Which of the following gaseous mixture does not follow Dalton's law of...
Text Solution
|
- Equal masses of methane and oxygen are mixed in an empty container at ...
Text Solution
|
- A box of 1 L capacity is divided into two equal compartments by a thin...
Text Solution
|
- If 10^(-4) dm^(3) of water is introduced into a 1.0 dm^(3) flask to 30...
Text Solution
|
- Dalton's law of partial pressures is not applicable to
Text Solution
|
- 56 g of nitrogen and 96 g of oxygen are mixed isothermaly and at a tot...
Text Solution
|
- The closed containers of the same capacity and at the same temperature...
Text Solution
|
- A jar contains a gas and a few drops of water at TK The pressure in th...
Text Solution
|
- In the equilibrium 2SO(2)(g)+O(2)(g)hArr2SO(3)(g) , the partial pressu...
Text Solution
|
- A gaseous mixture contains three gaseous A,B and C with a total number...
Text Solution
|