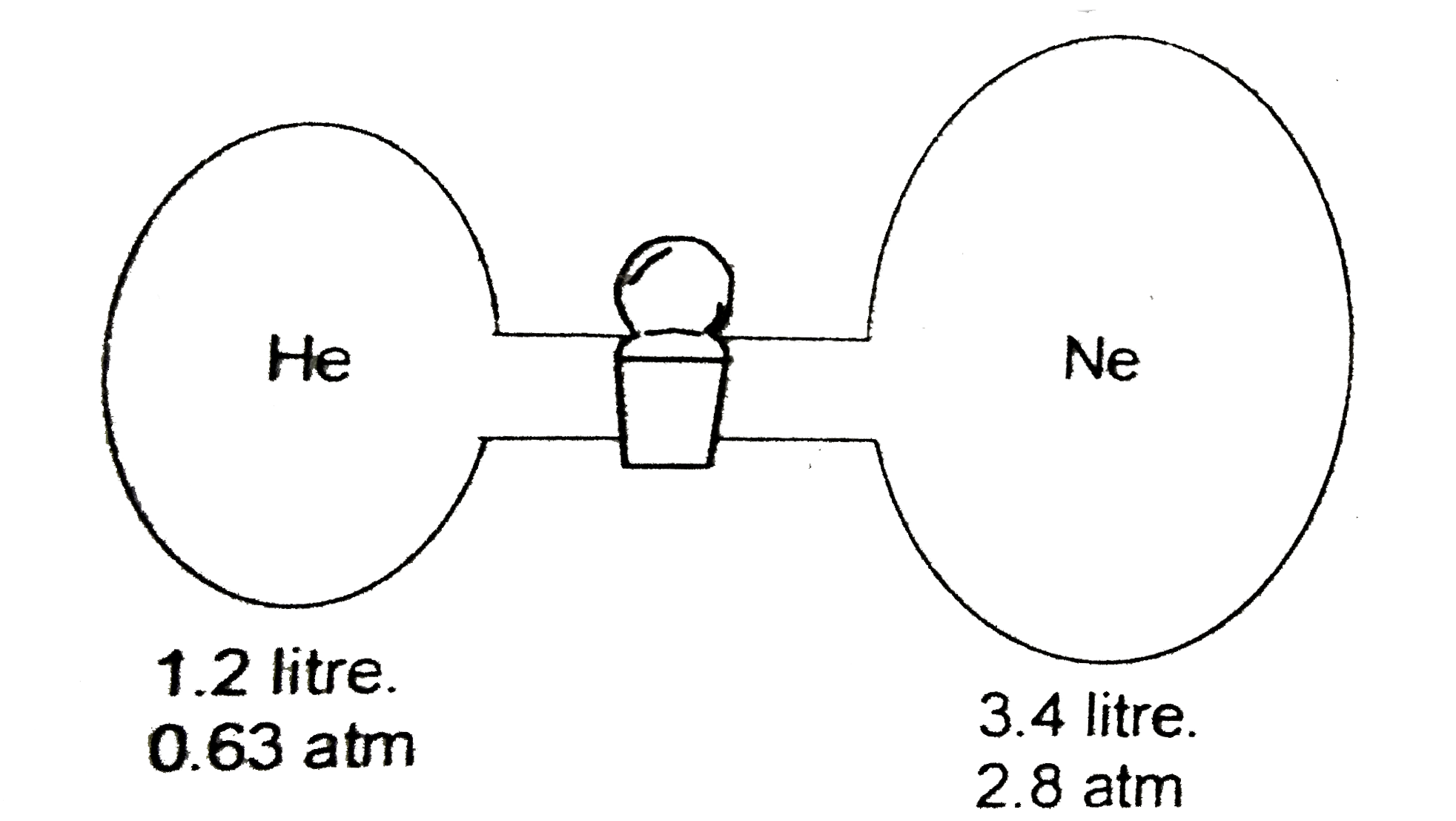
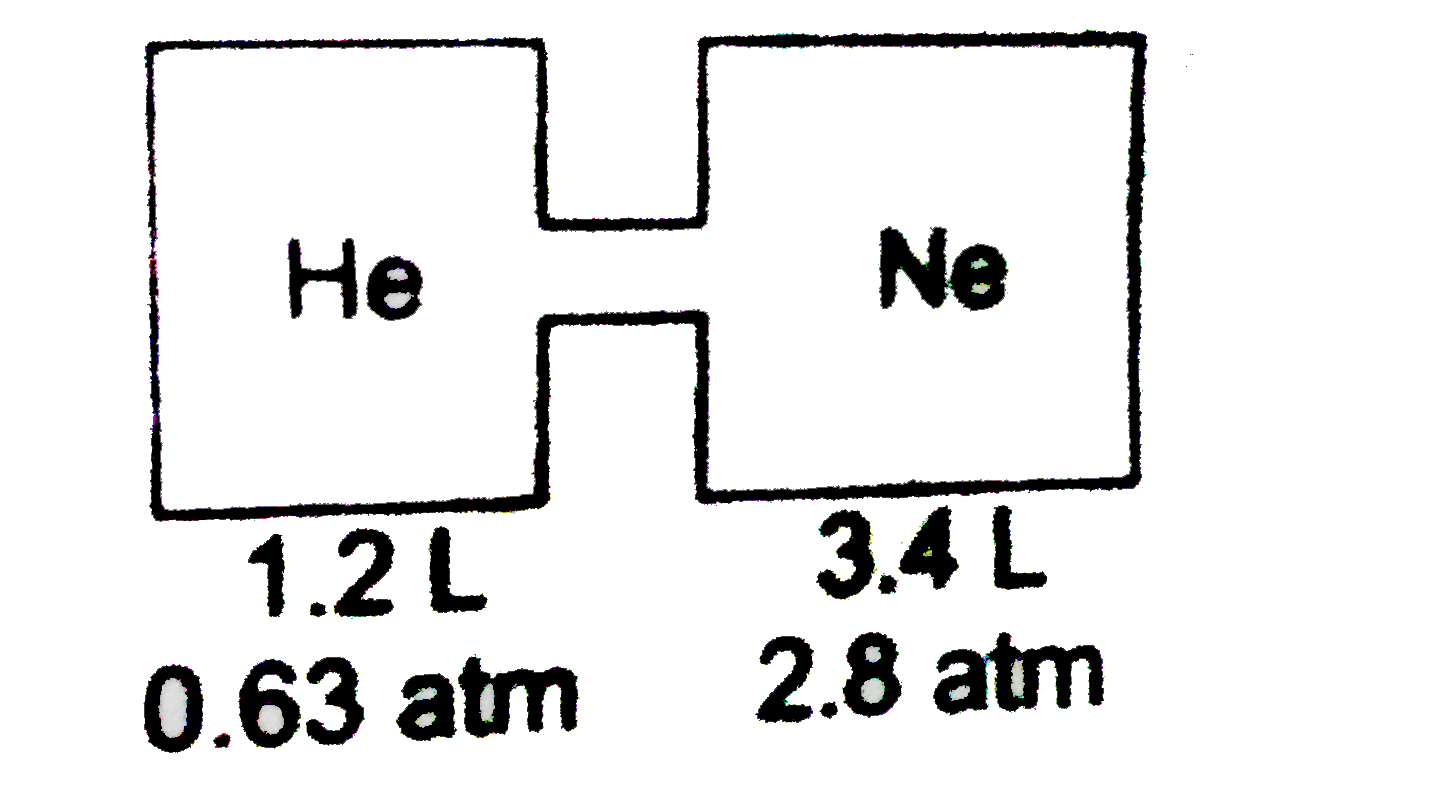A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE
NARENDRA AWASTHI|Exercise Level 1 (Q.151 To Q.176)|1 VideosGASEOUS STATE
NARENDRA AWASTHI|Exercise Level 2|1 VideosGASEOUS STATE
NARENDRA AWASTHI|Exercise Level 1 (Q.121 To Q.150)|1 VideosELECTROCHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|1 VideosIONIC EEQUILIBRIUM
NARENDRA AWASTHI|Exercise Assertin-Reason Type Questions|1 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-GASEOUS STATE-Exercise
- Dry ice is solid carbon dioxide. A 0.050 g sample of dry ice is placed...
Text Solution
|
- A mixture of helium of neon gases is collected over water at 28.0^(@)C...
Text Solution
|
- Consider the following apparatus. Calculate the partical pressure of H...
Text Solution
|
- Oxygen gas generated by the decomposition of potassium chlorate is col...
Text Solution
|
- The quantity (PV)/(k(B)T) represents the ( k(B):Boltzmann constant)
Text Solution
|
- Which of the following statements about kinetic energy (K.E.) is true?
Text Solution
|
- At what temperature most probable speed of SO(2) molecule have the sam...
Text Solution
|
- Which of the following is NOT a postulate of the kinetic molecular the...
Text Solution
|
- Which one of the following relationships when graphed does not give a ...
Text Solution
|
- Consider Three one -litre flasks labeled A,B and C filled with the gas...
Text Solution
|
- Which of the following statements is false?
Text Solution
|
- Which is not correct in terms of kinetic theory of gases?
Text Solution
|
- Two flasks A and B have equal volumes. A is maintained at 300 K and B ...
Text Solution
|
- Kinetic energy and pressure of a gas of unit volume are related as:
Text Solution
|
- Two flask A and B of equal volumes maintained at temperature 300K an...
Text Solution
|
- Which of the following change is observed occurs when a substance X is...
Text Solution
|
- A mixture of Ne and Ar kept in a closed vessel at 250 K has a total ...
Text Solution
|
- In two vessels of 1 litre each at athe same temperature 1g of H(2) and...
Text Solution
|
- Four particles have speed 2, 3, 4 and 5 cm/s respectively Their RMS sp...
Text Solution
|
- A gaseous mixture contains 4 molecules with a velocity of 6 cm sec^(-...
Text Solution
|