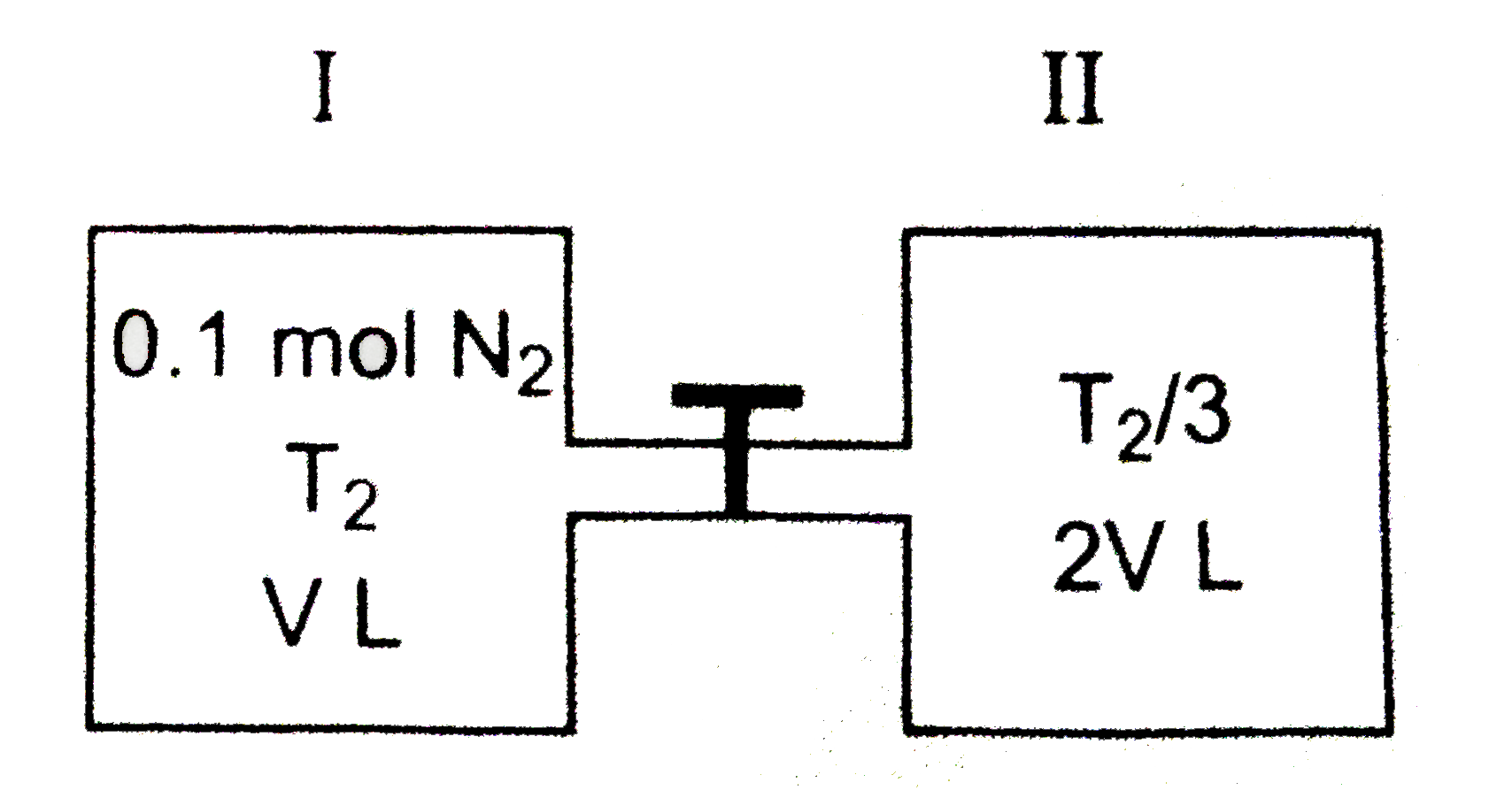A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE
NARENDRA AWASTHI|Exercise Level 1 (Q.151 To Q.176)|1 VideosGASEOUS STATE
NARENDRA AWASTHI|Exercise Level 2|1 VideosGASEOUS STATE
NARENDRA AWASTHI|Exercise Level 1 (Q.121 To Q.150)|1 VideosELECTROCHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|1 VideosIONIC EEQUILIBRIUM
NARENDRA AWASTHI|Exercise Assertin-Reason Type Questions|1 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-GASEOUS STATE-Exercise
- Consider the composite system, which is held at 300 k , shown in the f...
Text Solution
|
- 11 moles of N(2) and 12 moles of H(2) mixture reacted in 20 litre vess...
Text Solution
|
- Two vessels connected by a valve of negligible volume. One container (...
Text Solution
|
- A mixture of NH(3(g)) and N(2)H(4((g))) is placed in a sealed containe...
Text Solution
|
- Correct expression for density of an ideal gas mixture of two gases 1 ...
Text Solution
|
- Two closed vessels of equal volume containing air at pressure P(1) and...
Text Solution
|
- The following organic compound are popularly known by their common nam...
Text Solution
|
- The following organic compound are popularly known by their common nam...
Text Solution
|
- What is the density of wet air with 75% relative humidity at 1 atm and...
Text Solution
|
- 7 moles of a tetra-atomic non-linear gas 'A' at 10 atm and T K are mi...
Text Solution
|
- Three closed rigid vessels, A, B and C, which initially contain three ...
Text Solution
|
- 6xx10^(22) gas molecules each of mass 10^(-24)kg are taken in a vessel...
Text Solution
|
- A balloon of diameter 21 meter weight 100 kg. Calculate its pay-load, ...
Text Solution
|
- A given volume of ozonised oxygen (containing 60% oxygen by volume ) r...
Text Solution
|
- The following organic compound are popularly known by their common nam...
Text Solution
|
- The following organic compound are popularly known by their common nam...
Text Solution
|
- At low pressure, if RT=2sqrt(a.P), then the volume occupied by a real ...
Text Solution
|
- At room temperature following traction goes to completion 2AB(g)+B(2...
Text Solution
|
- A vessel of uniform cross-section of length 500 cm as shown in figure ...
Text Solution
|
- For a real gas (mol.mass =60) if density at critical point is 0.80g//c...
Text Solution
|