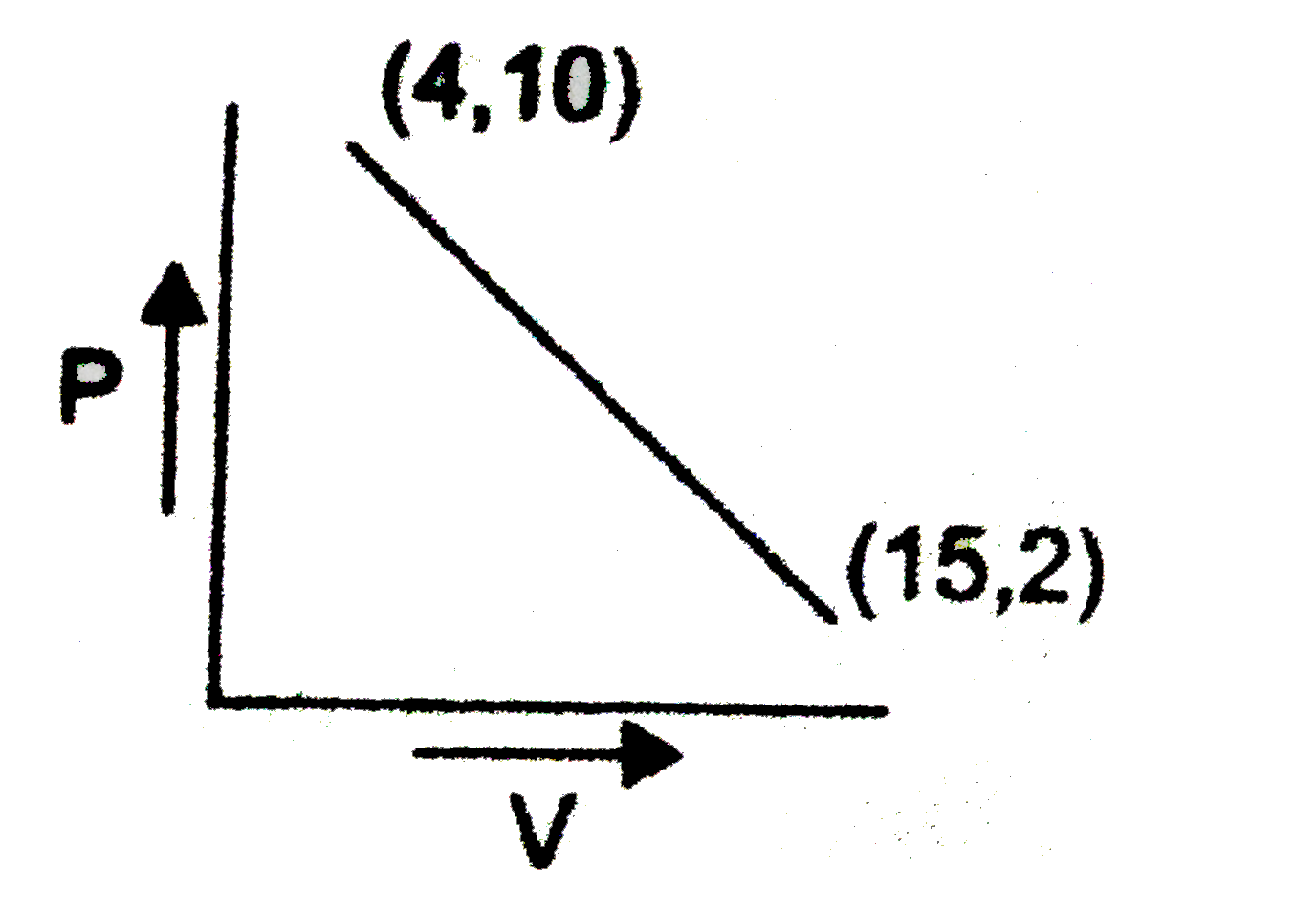
Equation of line
`P-2=(10-2)/(4-15)(V-15)`
`P-2=(8)/(11)(V-15)`
`P=2-(8V)/(11)+(15xx8)/(11)`
`P=((142)/(11)-(8V)/(11))`
`f(T)=(1)/(nR)((142V)/(11)-(8V^(2))/(11))`
`(d(F(T)))/(dV)=(1)/(nR)((142)/(11)-(8V^(2))/(11))=0`
`V=(142)/(11xx16)=8.875`
`P=(142)/(11)-(8)/(11)xx(8.875)/(16)=(71)/(11)`
`T_("Max")=(PV)/(nR)=(71)/(11)xx(142)/(16xx0.0821)=700`
`=7xx10^(2)K`