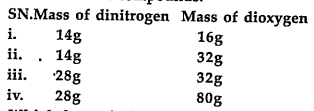Similar Questions
Explore conceptually related problems
Recommended Questions
- The following data are obtained when dinitrogen and dioxygen react tog...
Text Solution
|
- The following data are obtained when dinitrogen and dioxygen react to ...
Text Solution
|
- (A) When different compounds are formed by dioxygen reaction, the foll...
Text Solution
|
- The following data are obtained when dinitrogen and dioxygen react tog...
Text Solution
|
- The following data are obtained when dinitrogen and dioxygen react to ...
Text Solution
|
- (क) जब डाइनाइट्रोजन और डाइऑक्सीजन अभिक्रिया द्वारा भिन्न यौगिक बनाती ...
Text Solution
|
- 15.9 of copper sulphate and 10.6 g of sodium carbonate react together ...
Text Solution
|
- The following data are obtained when dinitrogen and dioxygen react tog...
Text Solution
|
- The experimental data for the reaction 2A + B2 to 2AB is The rat...
Text Solution
|