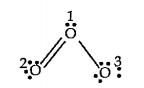
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The formal charge on three oxygen atoms of ozone molecules are respect...
Text Solution
|
- The formal charges on the three oxygen atoms in O(3) molecule are
Text Solution
|
- What will be the formal charges on the three oxygen atoms in ozone ?
Text Solution
|
- The formal charge on central oxygen atom in ozone is
Text Solution
|
- The formal charges of oxygen labelled 3,2,1 in ozone respectively are
Text Solution
|
- Calculate the formal charge on each oxygen atom in ozone.
Text Solution
|
- Calculate formal charge of the central atom in ozone molecule.
Text Solution
|
- The formal charges on the three oxygen atoms in O3 , molecule are
Text Solution
|
- In molecule, the formal charges of oxygen atoms 1,2,3 are respecti...
Text Solution
|