Similar Questions
Explore conceptually related problems
Recommended Questions
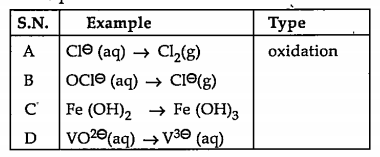
- Classify the following unbalanced half equations as oxidation and redu...
Text Solution
|
- In the unbalanced reaction, CrO(5)+SnCI(2)rarrCrO(4)^(2-)+SnCI(4), the...
Text Solution
|
- निम्नलिखित में कौन समीकरण असंतुलित हैं?
Text Solution
|
- इलेक्ट्रोडों पर होने वाली ऑक्सीकरण एवं अपयचन की अर्ध अभिक्रियाएं लिखिए...
Text Solution
|
- Unbalanced half equation ClO(3(aq))^(-) to ClO(2(aq))is
Text Solution
|
- The unbalanced half equation, CrO(4(aq))^(2-) to Cr(OH)((aq))^(-) is
Text Solution
|
- निम्नलिखित में कौन सा समीकरण असंतुलित है?
Text Solution
|
- In the following redox reactions, identify the oxidation half -reactio...
Text Solution
|
- What are oxidation and reduction half reactions?
Text Solution
|