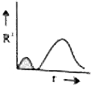
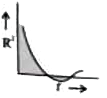
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-STRUCTURE OF ATOM -Level-1
- In photoelectric effect, the kinetic energy of photoelectrons increase...
Text Solution
|
- The number of spherical nodes in 3p orbitals are
Text Solution
|
- The probability density curve for 2s electron appears like
Text Solution
|
- Consider the following sets of quantum number Which of the foll...
Text Solution
|
- Amongst the elements with following electronic configurations, which o...
Text Solution
|
- Be^(2+) is isoelectronic with which of the following ions:
Text Solution
|
- The total number of orbitals present for principle quantum number, n=4...
Text Solution
|
- Which pair is of isoelectronic species?
Text Solution
|
- The emission spectrum of hydrogen discovered first and the region of t...
Text Solution
|
- Which is the correct order of increasing energy of the listed orbitals...
Text Solution
|
- If the first ionisation energy of H-atom is 13.6 eV, then the second i...
Text Solution
|
- The radius of 2nd Bohr orbit of hydrogen atom is :
Text Solution
|
- Calculate the energy in joule corresponding to light of wavelength 45 ...
Text Solution
|
- The uncertainty in the velocity of particle of mass 6.626xx10^(-28)kg ...
Text Solution
|
- The de-broglie wavelength associated with a mass of 1kg having K.E. 0....
Text Solution
|
- For an electron, if the uncertainty in velocity is Deltav, the uncerta...
Text Solution
|
- Which of the following statements do not form a part of Bohr.s model o...
Text Solution
|
- The total number of orbitals in a shell with principal quantum number ...
Text Solution
|
- According to the Bohr theory, which of the following transitions in th...
Text Solution
|
- In which transition, one quantum of energy is emitted
Text Solution
|