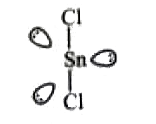
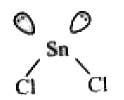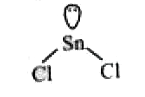A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
PHYSICS WALLAH|Exercise Level -2|48 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PHYSICS WALLAH|Exercise NEET Past 5 Years Questions |38 VideosBIOMOLECULES
PHYSICS WALLAH|Exercise NEET PAST 5 YEARS QUESTIONS|13 VideosCHEMISTRY IN EVERDAY LIFE
PHYSICS WALLAH|Exercise NEET PAST 5 YEARS QUESTIONS |6 Videos
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-CHEMICAL BONDING AND MOLECULAR STRUCTURE -NEET Past 5 Years Questions
- The atomic number of Sn is 50. The shape of gaseous SnCl2 molecule is
Text Solution
|
- Which of the following set of molecules will have zero diplole moment?
Text Solution
|
- Identify a molecule which does not exist.
Text Solution
|
- How many sp^2 hybridised carbon atoms and pi bonds respectively are pr...
Text Solution
|
- The potential energy(y) curve for H2 formation as a function of inrtnu...
Text Solution
|
- Identify the wrongly matched pair.
Text Solution
|
- Which of the following diatomic molecular species has only pi bonds ac...
Text Solution
|
- Identify the incorrect statement related to PCI5 from the following:
Text Solution
|
- Consider the following species CN^(-),CN^(-),NO and CN. Which one ...
Text Solution
|
- Which of the following pairs of species have the same bond order ?
Text Solution
|
- The species, having bond angles of 120^@ is
Text Solution
|
- which one of the following ions is not tetrahedral in shape ?
Text Solution
|
- Which of the following hydrides has the largent bond angle ?
Text Solution
|
- Which one of the following compounds shows the presence of intramolecu...
Text Solution
|
- In which of the following moleucles, all atoms are coplanar?
Text Solution
|
- Among the following which one is a wrong statement?
Text Solution
|
- The hybridisations of atomic orbitals of nitrogen in NO2, NO3^(-) and ...
Text Solution
|
- Which of the following pairs of ions is isoelectronic and isostructur...
Text Solution
|
- Consider the molecules CH(4), NH3 and H2O What of the given statement ...
Text Solution
|
- Predict the correct order omong the following:
Text Solution
|
- Which of the following set of molecules will have zero dipole moment?
Text Solution
|