A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
PHYSICS WALLAH|Exercise NEET Past 5 Years Questions |38 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PHYSICS WALLAH|Exercise NEET Past 5 Years Questions |38 VideosBIOMOLECULES
PHYSICS WALLAH|Exercise NEET PAST 5 YEARS QUESTIONS|13 VideosCHEMISTRY IN EVERDAY LIFE
PHYSICS WALLAH|Exercise NEET PAST 5 YEARS QUESTIONS |6 Videos
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-CHEMICAL BONDING AND MOLECULAR STRUCTURE -Level -2
- The ion which is not tetrahedral in shape :
Text Solution
|
- In a regular octahedral molecule MX(6) the number of X - M - X bonds ...
Text Solution
|
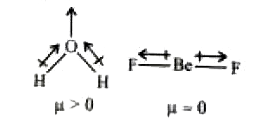
- H2O is dipolar, whereas BeF2 is not. It is because
Text Solution
|
- Which hydrogen is most polar ?
Text Solution
|
- The correct order of dipole moment is :
Text Solution
|
- The molecule that deviates from octet rule is
Text Solution
|
- The molecules which has zero dipole moment is:
Text Solution
|
- The number of anti-bonding electrons pairs in molecular ion on the bas...
Text Solution
|
- Which one in the following is not the resonating structure of CO2 ?
Text Solution
|
- Which one of the following can not exist on the basis of molecular orb...
Text Solution
|
- In which of the following set, the values of bond orders will be 2.5 ?
Text Solution
|
- Number of antibonding electrons in N2 is:
Text Solution
|
- An ionic bond A B is most likely to be formed when:
Text Solution
|
- Which one of the following has the shortest carbon carbon bond length?
Text Solution
|
- Which of the following shows the highest lattice energy?
Text Solution
|
- Which one of the following combination of ion will have highest polari...
Text Solution
|
- Correct order of covalent character of alkaline earth metal chloride i...
Text Solution
|
- dpi -ppi bond is present in
Text Solution
|
- In BrF3 molecule, the lone pairs occupy equatorial positions to minim...
Text Solution
|
- The correct order towards bond angle is:
Text Solution
|