A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-ELECTROCHEMISTRY-NEET PAST 5 YEARS QUESTIONS
- For a cell involving one electron E(cell)^(0)=0.59V and 298K, the equi...
Text Solution
|
- For the cell reaction: 2Fe^(3+)(aq)+2l^(-)(aq)to2Fe^(2+)(aq)+l(2)(aq...
Text Solution
|
- Consider the change in oxidation state of Bromine corredponding to dif...
Text Solution
|
- The emf of a Daniell cell at 298 K is E(1) Zn|ZnSO(4)(0.01 M)||CuSO(...
Text Solution
|
- Given that wedge(m)^(@) = 133.45 cm^(2) mol^(-1) (AgNO(3)) wedge(m)^(@...
Text Solution
|
- The zinc/silver oxide cell is used in electric current reaction is as ...
Text Solution
|
- During the electrolysis of molten sodium chloride, the time required t...
Text Solution
|
- Zine can be coated on iron to produce galvanize3d iron but the reverse...
Text Solution
|
- The number of electrons delivered at the cathode during electrolysis b...
Text Solution
|
- If the E^(@) for a given reaction has a negative value, then which of ...
Text Solution
|
- The molar conductivity of a 0.5 mol dm^(-1) solution with electrolytic...
Text Solution
|
- The pressure of H(2) required to make the potential of H(2)-electrode ...
Text Solution
|
- On elctrolysis of dil sulphuric acid using Platinum (Pt) electrode the...
Text Solution
|
- The number of Faraday.s (F) required to produce 20 g of calcium from C...
Text Solution
|
- Identify the reaction from following having top position is EMF series...
Text Solution
|
- In a typical fuel cell, the reactants (R) and product (P) are:
Text Solution
|
- For a cell involving one electron E(cell)^(0)=0.59V and 298K, the equi...
Text Solution
|
- For the cell reaction 2Fe^(2+)(aq) + 2I^(-)(aq) to 2Fe^(2+)(aq) + I(...
Text Solution
|
- Consider the change in oxidation state of Bromine corresponding to dif...
Text Solution
|
- In the electrochemical cell: Zn|ZnSO(4)(0.01 M)||CuSO(4)(1.0 M)|Cu, ...
Text Solution
|
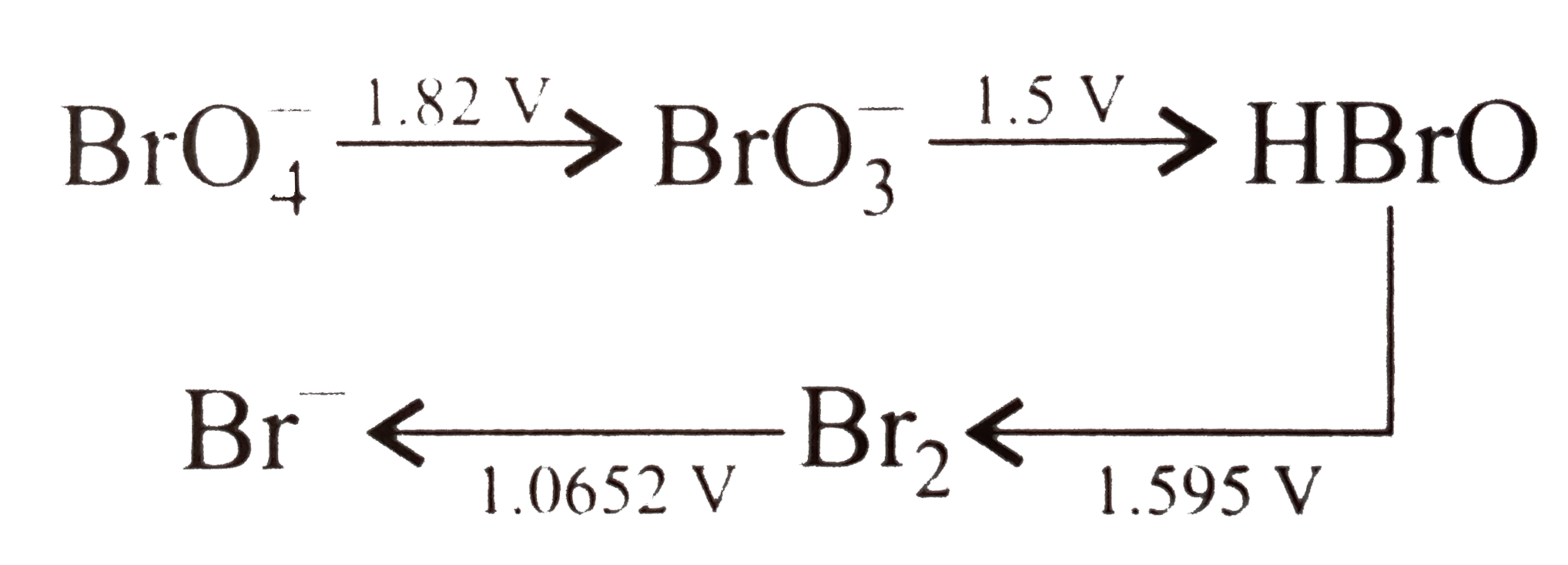 .
.