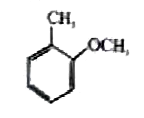
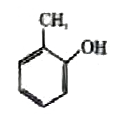
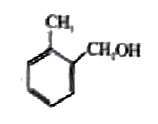
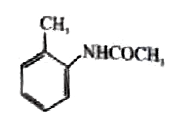
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-HYDROCARBONS-LEVEL-1
- Which of the following reacts with KMnO(4) but does not react with tol...
Text Solution
|
- The dihedral angle between the hydrogen atoms of two methyl groups in ...
Text Solution
|
- Which one is the most reactive towards electrophilic reagent?
Text Solution
|
- The following reaction is known as: C(6)H(6)+CH(3)Cl overset(AlCl(3)...
Text Solution
|
- In the following reaction, the major product is :
Text Solution
|
- Complete the following reaction
Text Solution
|
- The final major product of the following reaction is : CH(3)-CH(2)-C...
Text Solution
|
- Which of the following species is an intermediate in the reaction show...
Text Solution
|
- Maleic acid reacts with Br(2)//C Cl(4) The production
Text Solution
|
- Sodium salt of the following acid which is needed for the preparation ...
Text Solution
|
- Which of the following is the most stable free radical?
Text Solution
|
- H(3)C-C-=CH overset(H(2)O,H(4)SO(4))underset(HgSO(4))to underset(A)("i...
Text Solution
|
- Which compound cannot be formed from Wurtz
Text Solution
|
- Which of the following is called marsh gas?
Text Solution
|
- Which one is the correct order of acidity ?
Text Solution
|
- The case of dehydrohalogenation for different halogensis in the order
Text Solution
|
- Which of the following is the predominant product in the reaction of H...
Text Solution
|
- The reaction represents : C(12)H(26) to C(6) H(12)+C(6)H(14)
Text Solution
|
- Ethylene can be separated from acetylene by passing the mixture throug...
Text Solution
|
- Ozonolysis products of 2-pentyne after decomposition of ozonide with w...
Text Solution
|