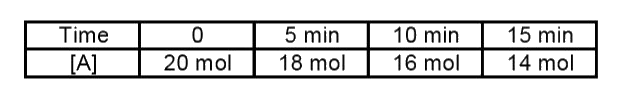
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-MOLECULARITY -LEVEL-2
- K for a zero order reaction is 2 xx 10^(-2) "mol L"^(-1) "sec"^(-1). I...
Text Solution
|
- In a certain reaction, 10% of the reactant decomposes in one hour, 20%...
Text Solution
|
- The given reaction 2 "FeCI"3 + "SnCI"2 to 2"FeCI"2 + "SnCI"4 is an...
Text Solution
|
- The rate constant for a reaction is ("In 2")/(10) " min"^(-1). What wi...
Text Solution
|
- For the reaction A rarr" Product, "-(d[A])/(dt)=k and at different tim...
Text Solution
|
- Consider the reaction 2A(g) rarr 3B(g) + C(g). Starting with pure A in...
Text Solution
|
- Let there be as first-order reaction of th type. A to B + C Let us ass...
Text Solution
|
- Rate of which reactions increases with temperature:
Text Solution
|
- The rate constant, the activation energy and the Arrhenius parameter o...
Text Solution
|
- The activation energy for the forward reaction X rarr Y is 60 kJ mol^(...
Text Solution
|
- For a given reaction, energy of activation for forward reaction (E(af)...
Text Solution
|
- In the reaction NH(4)NO(2)(aq.) rarr N(2)(g) + 2H(2)O(l) the volume of...
Text Solution
|
- In a first order reaction A to B, if k is rate constant and initial co...
Text Solution
|
- For a reaction following n^(th) order kinetics, the half life (t(1//2)...
Text Solution
|
- For the reaction, 2N(2)O(5)to4NO(2)+O(2) rate and rate constant are 1....
Text Solution
|
- 3A rarr B + C It would be a zero order reaction when :
Text Solution
|
- If the rate of the reaction is equal to the rate constant , the order ...
Text Solution
|
- In a first order reaction A to B, if k is rate constant and initial co...
Text Solution
|
- The rate constant k(1) and k(2) for two different reactions are 10^(16...
Text Solution
|
- Which one of the following statements for the reaction is incorrect?
Text Solution
|