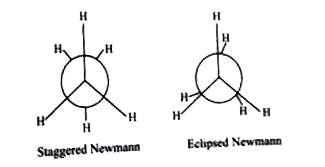
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-HYDROCARBONS-NEET PAST 5 YEARS QUESTIONS
- The correct statement regarding the conformation is
Text Solution
|
- The compound that will react most readily with gaseous bromine has the...
Text Solution
|
- In pyrole, the electron density is maximum on
Text Solution
|
- In the given reaction, the product P is :
Text Solution
|
- The correct statement regarding the comparison of and eclipsed conform...
Text Solution
|
- For the following reaction : (A) CH(3)CH(2)CH(2)Br+KOH to CH(3)CH=CH...
Text Solution
|
- Which of the following is a free radical substitution reaction?
Text Solution
|
- Which of the following compounds is most reactive in electrophilic aro...
Text Solution
|
- The number of sigma (sigma) and pi(pi) bonds in pent-2-en-4-yne is:
Text Solution
|
- Among the following the reaction that produce through an electrophilic...
Text Solution
|
- Hydrocarbon (A) reacts with bromine by substitution to form an alkyl b...
Text Solution
|
- The compound C(7)H(8) undergoes the following reactions : C(7)H(8) o...
Text Solution
|
- Which one is the correct order of acidity ?
Text Solution
|
- The correct statement regarding the conformation is
Text Solution
|
- The compound that will react most readily with gaseous bromine has the...
Text Solution
|
- Which of the following can beused as the halide component for friedel-...
Text Solution
|
- In pyrole, the electron density is maximum on
Text Solution
|
- In the given reaction, the product P is :
Text Solution
|
- The correct statement the comparison of staggered and eclipsed conform...
Text Solution
|
- For the following reaction : (A) CH(3)CH(2)CH(2)Br+KOH to CH(3)CH=CH...
Text Solution
|