Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMISTRY-2017
ICSE|Exercise Question (Answer the following)|4 VideosCHEMISTRY-2017
ICSE|Exercise Question (Answer the following)|4 VideosCHEMISTRY IN EVERYDAY LIFE
ICSE|Exercise EXERCISE (PART - II (DESCRIPTIVE QUESTIONS) (LONG ANSWER QUESTIONS))|19 VideosCHEMISTRY-2018
ICSE|Exercise Question (Answer the following question) |5 Videos
Similar Questions
Explore conceptually related problems
ICSE-CHEMISTRY-2017-Question (Answer the following questions)
- Answer the following questions : Why the freezing point depression (...
Text Solution
|
- What do you understand by the order of a reaction ? Identify the react...
Text Solution
|
- Specific conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm...
Text Solution
|
- Name the order of reaction which proceeds with a uniform rate througho...
Text Solution
|
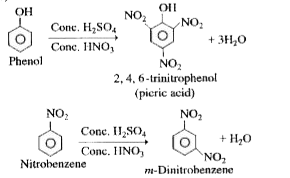
- What are the products formed when phenol and nitrobenzene are treated ...
Text Solution
|